当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Heck‐Matsuda Reactions of Spirocyclopentenyl Hydantoins Directed by Non‐Covalent Interactions: Total Synthesis of the (S,S)‐VPC01091
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-19 , DOI: 10.1002/adsc.202000443 Valdeir C. Oliveira 1 , Juliana M. Oliveira 1 , Vitor H. Menezes da Silva 1 , Ismat U. Khan 2 , Carlos Roque D. Correia 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-19 , DOI: 10.1002/adsc.202000443 Valdeir C. Oliveira 1 , Juliana M. Oliveira 1 , Vitor H. Menezes da Silva 1 , Ismat U. Khan 2 , Carlos Roque D. Correia 1
Affiliation
|
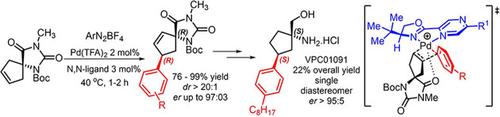
A highly efficient Heck‐Matsuda desymmetrization of unsaturated spirohydantoins directed by non‐covalent interactions, which allows the construction of two simultaneous stereogenic centers, including a trisubstituted quaternary one, is described. This Heck arylation permitted a novel enantioselective total synthesis of the S1PR1 agonist (also an S1PR3 antagonist) compound VPC01091, a potential drug for the treatment of multiple sclerosis. The broad scope of these enantioselective Heck‐Matsuda protocol provided several arylated spiro systems in yields ranging from 76% to 99%, with enantiomeric ratios (er) up to 97:3, and diastereoselectivities (dr) of >20:1 in all cases studied. The method uses only 2% of Pd(TFA)2 and 3 mol% of the chiral N,N‐ligand Pyrabox in short reaction times of 1–2 h. These enantioselective Heck arylations can also be carried out at the gram scale in high yields with no erosion of their diastereoselectivity or enantioselectivity. The key spiro Heck products (R,R)‐21 and (R,R)‐27 bearing an aryl iodide moiety or an aryl n‐octyl moiety were employed as starting materials for the total enantioselective syntheses of the (S,S)‐VPC01091, in overall yields of 20% and 22% respectively after 10 or 9 steps from the starting spirohydantoin, with an er>95:5. Computational analysis of the enantioselective Heck‐Matsuda desymmetrization supports the rationale involving a key non‐covalent interaction between the imide carbonyl of the spirohydantoin and the cationic palladium bounded to the chiral N,N‐ligand.
中文翻译:

非共价相互作用指导的螺环戊烯基乙内酰脲对映体选择性Heck-Matsuda反应:(S,S)-VPC01091的全合成
描述了一种由非共价相互作用指导的不饱和螺乙内酰脲的高效Heck-Matsuda去对称化,它可以构建两个同时的立体异构中心,包括一个三取代的季铵盐中心。这种Heck芳基化反应可实现S1PR1激动剂(也是S1PR3拮抗剂)化合物VPC01091的新型对映选择性全合成,该化合物可用于治疗多发性硬化症。这些对映选择性Heck-Matsuda方案的广泛范围提供了几种芳基化螺环系统,收率范围从76%到99%,对映体比率(er)高达97:3,非对映选择性(dr)在所有情况下均> 20:1研究。该方法仅使用2%的Pd(TFA)2和3 mol%的手性N,N-配体Pyrabox在1-2小时的短反应时间内。这些对映选择性的Heck芳基化反应也可以以克规模以高收率进行,而不会损害它们的非对映选择性或对映选择性。带有芳基碘化物部分或芳基正辛基部分的主要Spiro Heck产物(R,R)‐21和(R,R)‐27被用作(S,S)‐的全部对映选择性合成的原料VPC01091,在20%和之后从起始spirohydantoin 10个或9个步骤22%的总产率,与ER> 95:5。对映选择性Heck-Matsuda脱对称的计算分析支持了基本原理,涉及螺乙内酰脲的酰亚胺羰基和与手性N,N-配体结合的阳离子钯之间的关键非共价相互作用。
更新日期:2020-08-19
中文翻译:

非共价相互作用指导的螺环戊烯基乙内酰脲对映体选择性Heck-Matsuda反应:(S,S)-VPC01091的全合成
描述了一种由非共价相互作用指导的不饱和螺乙内酰脲的高效Heck-Matsuda去对称化,它可以构建两个同时的立体异构中心,包括一个三取代的季铵盐中心。这种Heck芳基化反应可实现S1PR1激动剂(也是S1PR3拮抗剂)化合物VPC01091的新型对映选择性全合成,该化合物可用于治疗多发性硬化症。这些对映选择性Heck-Matsuda方案的广泛范围提供了几种芳基化螺环系统,收率范围从76%到99%,对映体比率(er)高达97:3,非对映选择性(dr)在所有情况下均> 20:1研究。该方法仅使用2%的Pd(TFA)2和3 mol%的手性N,N-配体Pyrabox在1-2小时的短反应时间内。这些对映选择性的Heck芳基化反应也可以以克规模以高收率进行,而不会损害它们的非对映选择性或对映选择性。带有芳基碘化物部分或芳基正辛基部分的主要Spiro Heck产物(R,R)‐21和(R,R)‐27被用作(S,S)‐的全部对映选择性合成的原料VPC01091,在20%和之后从起始spirohydantoin 10个或9个步骤22%的总产率,与ER> 95:5。对映选择性Heck-Matsuda脱对称的计算分析支持了基本原理,涉及螺乙内酰脲的酰亚胺羰基和与手性N,N-配体结合的阳离子钯之间的关键非共价相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号