Molecular Catalysis ( IF 4.6 ) Pub Date : 2020-06-22 , DOI: 10.1016/j.mcat.2020.111067 Rahul Kumar Rajmani Singh , Tolga N.V. Karsili , Radhey Srivastava
|
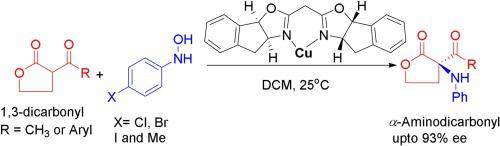
We report a novel and efficient Cu-catalyzed direct asymmetric amination of tertiary β-carbonyl compounds using aryl hydroxylamine as electrophilic nitrogen donor. The process facilitates the convenient and direct synthesis of chiral α-amino carbonyl derivatives, without the need for any post-reaction manipulation. This method reveals an effective strategy for the synthesis of enantioenriched α-CH aminated derivatives which is hitherto challenging. The choice of the robust chiral indabox ligand was ascertained to be very crucial for the desired enantioselectivity in the contemporary transformation. The reaction mechanism is fully supported by ab initio electronic structure calculations. The reaction is facile, efficient and performs well at room temperature with an enantiomeric excess (ee) up to 93 %.
中文翻译:

铜催化β-二羰基衍生物与芳基羟胺的对映选择性直接α - CH胺化反应及其机理研究
我们报告了新型和高效的铜催化的使用芳基羟胺作为亲电氮供体的叔β-羰基化合物的直接不对称胺化。该方法促进了手性α-氨基羰基衍生物的方便和直接合成,而无需任何后反应操作。该方法揭示了合成对映体富集的α- C H胺化衍生物的有效策略,该策略迄今仍具有挑战性。确定了对于现代转化中所需的对映选择性而言,至关重要的手性indabox配体的选择非常关键。从头开始完全支持反应机理电子结构计算。该反应容易,有效并且在室温下表现良好,对映体过量(ee)高达93%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号