Journal of Neuroscience Methods ( IF 3 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.jneumeth.2020.108807 Julie Fourneau 1 , Caroline Cieniewski-Bernard 1 , Marie-Hélène Canu 1 , Sophie Duban-Deweer 2 , Johann Hachani 2 , Bruno Bastide 1 , Erwan Dupont 1
|
Background
Several studies have shown the importance of phosphorylation, O-GlcNAcylation and their interplay in neuronal processes.
New method
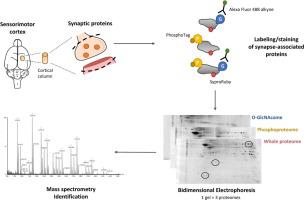
To get understanding about molecular mechanisms of synaptic plasticity, we performed a preparation of synaptic protein-enriched fraction on a small sample of rat sensorimotor cortex. We then optimized a multiplexed proteomic strategy to detect O-GlcNAcylated proteins, phosphoproteins, and the whole proteome within the same bidimensional gel. We compared different protocols (solubilisation buffer, reticulation and composition of the gel, migration buffer) to optimize separating conditions for 2D-gel electrophoresis of synaptic proteins. The O-GlcNAcome was revealed using Click chemistry and the azide–alkyne cycloaddition of a fluorophore on O-GlcNAc moieties. The phosphoproteome was detected by Phospho-Tag staining, while the whole proteome was visualized through SYPRORuby staining.
Results
This method permitted, after sequential image acquisition, the direct in-gel detection of O-GlcNAcome, phosphoproteome, and whole proteome of synapse-associated proteins.
Conclusion
This original method of differential proteomic analysis will permit to identify key markers of synaptic plasticity that are O-GlcNAcylated and/or phosphorylated, and their molecular regulations in neuronal processes.
中文翻译:

2-DE的优化和大鼠感觉运动皮层内突触相关蛋白的O-GlcNAcome,磷酸化蛋白质组和整个蛋白质组方案的多重检测。
背景
多项研究表明,磷酸化,O-GlcNAcylation及其在神经元过程中的相互作用非常重要。
新方法
为了了解突触可塑性的分子机制,我们在少量大鼠感觉运动皮层样品上进行了突触蛋白富集级分的制备。然后,我们优化了多重蛋白质组学策略,以在同一二维凝胶中检测O-GlcNAcylated蛋白,磷蛋白和整个蛋白质组。我们比较了不同的协议(增溶缓冲液,凝胶的网状结构和组成,迁移缓冲液),以优化突触蛋白2D凝胶电泳的分离条件。使用Click化学和O-GlcNAc部分上荧光团的叠氮化物-炔烃环加成反应揭示了O-GlcNAcome。磷酸化蛋白质组通过Phospho-Tag染色检测,而整个蛋白质组通过SYPRORuby染色可视化。
结果
在连续图像采集后,该方法可以直接在凝胶中检测O-GlcNAcome,磷酸化蛋白质组和突触相关蛋白的整个蛋白质组。
结论
这种原始的差异蛋白质组学分析方法将允许鉴定被O-GlcNA酰化和/或磷酸化的突触可塑性的关键标志物,以及它们在神经元过程中的分子调控。



























 京公网安备 11010802027423号
京公网安备 11010802027423号