Journal of Biomedical informatics ( IF 4.5 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.jbi.2020.103491 Lauren Houston 1 , Ping Yu 2 , Allison Martin 3 , Yasmine Probst 1
|
Introduction
Clinical research is vital in the discovery of new medical knowledge and reducing disease risk in humans. In clinical research poor data quality is one of the major problems, affecting data integrity and the generalisability of the research findings. To achieve high quality data, guidance needs to be provided to clinical studies on the collection, processing and handling of data. However, clinical trials are implementing ad hoc, pragmatic approaches to ensure data quality. This study aims to explore the procedures for ensuring data quality in Australian clinical research studies.
Material and methods
We conducted a national cross-sectional, mixed-mode multi-contact (postal letter and e-mail) web-based survey of clinical researchers associated with clinical studies listed on the Australian and New Zealand Clinical Trials Registry.
Results
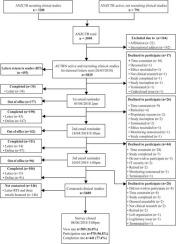
Of the 3689 clinical studies contacted, 589 (16%) responded, 570 (97%) consented and 441 (77%) completed the survey. 67% clinical studies reported following national and/or international guidelines for data monitoring, with the National Statement (86%) and Good Clinical Practice Guidelines (55%) most common. Source data were most likely to be recorded on one instrument (46%), of which paper (77%) being most common. 46.4% studies did not use data management software and 55% monitored data via traditional approaches (e.g. source data verification). Training on data quality was only provided to less than half of the staff responsible for data entry (43.9%) and data monitoring (37.5%). Regression analysis on 179 (33%) respondents found a borderline significant association between intervention trials and a definition for protocol deviation and/or violation (odds 3.065, p = 0.096). This may suggest when clinical trials are provided with additional guidance and resources, they are more likely to implement required procedures. Statistical strength of the full regression model was not significant χ2 (13, 179) = 15.827, p = 0.259.
Conclusion
Small single-site academic clinical studies implemented ad hoc procedures to ensure data quality. Education and training are required to promote standardised practices to ensure data quality in small scale clinical trials.
中文翻译:

临床研究数据质量监测中的异质性:国家调查。
介绍
临床研究对于发现新的医学知识并降低人类患病风险至关重要。在临床研究中,不良数据质量是主要问题之一,影响数据完整性和研究结果的普遍性。为了获得高质量的数据,需要为临床研究提供有关数据收集,处理和处理的指南。但是,临床试验正在采用临时,实用的方法来确保数据质量。这项研究旨在探索确保澳大利亚临床研究数据质量的程序。
材料与方法
我们对与澳大利亚和新西兰临床试验注册中心中列出的临床研究相关的临床研究人员进行了全国性的,基于混合模式的多接触(邮件和电子邮件)网络交叉调查。
结果
在所访问的3689项临床研究中,有589项(16%)得到了答复,有570项(97%)得到了同意,有441项(77%)完成了调查。67%的临床研究报告遵循国家和/或国际数据监测指南,最常见的是国家声明(86%)和良好临床实践指南(55%)。源数据最有可能记录在一种仪器上(46%),其中纸张(77%)最为常见。46.4%的研究未使用数据管理软件,而55%的研究通过传统方法(例如源数据验证)监控了数据。数据质量方面的培训仅提供给负责数据录入(43.9%)和数据监视(37.5%)的工作人员不到一半。对179名(33%)的受访者进行的回归分析发现,干预试验与方案偏离和/或违反的定义之间存在明显的显着关联(奇数3.065,p = 0.096)。这可能表明,当为临床试验提供更多指导和资源时,它们更有可能实施所需的程序。完全回归模型的统计强度不显着χ2(13,179)= 15.827,p = 0.259。
结论
小型单站点学术临床研究实施了临时程序以确保数据质量。需要进行教育和培训以促进标准化做法,以确保小规模临床试验中的数据质量。


























 京公网安备 11010802027423号
京公网安备 11010802027423号