Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.bmcl.2020.127361 Hiroshi Nakamura 1 , Shingo Fujioka 2 , Takashi Terui 2 , Satoshi Okuda 2 , Kentaro Kondo 2 , Yoshinori Tamatani 2 , Yusuke Akagi 2 , Yasumasa Komoda 2 , Wataru Kinoshita 2 , Soichiro Ito 2 , Kimiya Maeda 2 , Yutaka Ukaji 3 , Takashi Inaba 2
|
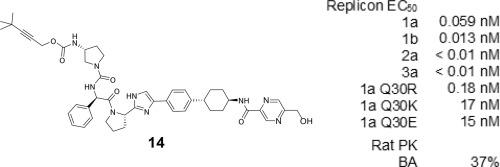
A novel unsymmetrical structural class of orally bioavailable hepatitis C virus (HCV) nonstructural 5A protein (NS5A) inhibitors has been generated by improving both the solubility and membrane permeability of the lead compound found in our previous work. The representative compound 14, with a 5-hydroxymethylpyrazine group and a 3-t-butylpropargyl group on each side of the molecule, exhibited the best oral bioavailability in this study, inhibiting not only the HCV genotype 1a, 1b, 2a, and 3a replicons with EC50 values in the picomolar range, but also inhibited 1a Q30 mutants induced by launched symmetrical inhibitors with EC50 values in the low nanomolar range.
中文翻译:

非对称结构类别的口服生物可利用的 HCV NS5A 抑制剂。
通过提高我们之前工作中发现的先导化合物的溶解度和膜通透性,已经产生了一种新型的不对称结构类口服生物可利用的丙型肝炎病毒 (HCV) 非结构性 5A 蛋白 (NS5A) 抑制剂。具有代表性的化合物14,在分子的每一侧具有 5-羟甲基吡嗪基团和 3-叔丁基炔丙基基团,在本研究中表现出最佳的口服生物利用度,不仅抑制 HCV 基因型 1a、1b、2a 和 3a 复制子EC 50值在皮摩尔范围内,但也抑制了由 EC 50值在低纳摩尔范围内的对称抑制剂诱导的 1a Q30 突变体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号