Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.bmc.2020.115600 Thales Kronenberger 1 , Glaucio Monteiro Ferreira 2 , Alfredo Danilo Ferreira de Souza 3 , Soraya da Silva Santos 4 , Antti Poso 1 , João Augusto Ribeiro 5 , Maurício Temotheo Tavares 3 , Fernando Rogério Pavan 6 , Gustavo Henrique Goulart Trossini 7 , Marcio Vinícius Bertacine Dias 8 , Roberto Parise-Filho 3
|
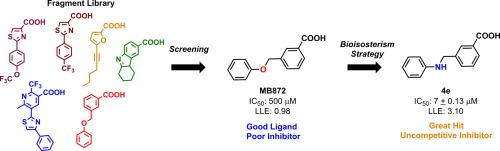
The enzyme dihydrofolate reductase from M. tuberculosis (MtDHFR) has a high unexploited potential to be a target for new drugs against tuberculosis (TB), due to its importance for pathogen survival. Preliminary studies have obtained fragment-like molecules with low affinity to MtDHFR which can potentially become lead compounds. Taking this into account, the fragment MB872 was used as a prototype for analogue development by bioisosterism/retro-bioisosterism, which resulted in 20 new substituted 3-benzoic acid derivatives. Compounds were active against MtDHFR, with IC50 values ranging from 7 to 40 μM, where compound 4e not only had the best inhibitory activity (IC50 = 7 μM), but also was 71-fold more active than the original fragment MB872. The 4e inhibition kinetics indicated an uncompetitive mechanism, which was supported by molecular modeling which suggested that the compounds can access an independent backpocket from the substrate and competitive inhibitors. Thus, based on these results, substituted 3-benzoic acid derivatives have strong potential to be developed as novel MtDHFR inhibitors and also anti-TB agents.
中文翻译:

新型取代的3-苯甲酸衍生物作为MtDHFR抑制剂的设计,合成和生物学活性。
结核分枝杆菌的二氢叶酸还原酶(Mt DHFR)具有很高的未被开发的潜力,因为它对病原体的生存很重要,因此成为抗结核新药(TB)的靶标。初步研究已获得了与Mt DHFR亲和力低的片段状分子,这些分子可能成为先导化合物。考虑到这一点,片段MB872被用作通过生物等位基因/反生物等位基因发展类似物的原型,其产生了20个新的取代的3-苯甲酸衍生物。化合物具有抗Mt DHFR的活性,IC 50值为7至40μM,其中化合物4e不仅具有最佳的抑制活性(IC 50 = 7μM),而且活性比原始片段MB872高71倍。在图4e抑制动力学表示的无竞争的机制,这是由这表明,这些化合物可以从基板与竞争性抑制剂访问一个独立的backpocket分子建模支持。因此,基于这些结果,取代的3-苯甲酸衍生物具有被开发为新型的Mt DHFR抑制剂以及抗TB剂的强大潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号