Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nuclear delivery of dual anticancer drug-based nanomedicine constructed by cisplatinum-induced peptide self-assembly.
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0nr00143k Tengyan Xu 1 , Chunhui Liang , Debin Zheng , Xiaorong Yan , Yaoxia Chen , Yumiao Chen , Xinxin Li , Yang Shi , Ling Wang , Zhimou Yang
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0nr00143k Tengyan Xu 1 , Chunhui Liang , Debin Zheng , Xiaorong Yan , Yaoxia Chen , Yumiao Chen , Xinxin Li , Yang Shi , Ling Wang , Zhimou Yang
Affiliation
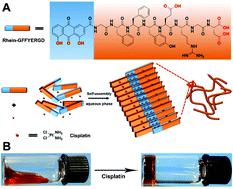
|
Nuclear delivery of anticancer drugs, particularly dual complementary anticancer drugs, can significantly improve chemotherapy efficacy. However, successful examples are rare. We reported a novel dual anticancer drug-based nanomedicine with nuclear accumulation properties. The nanomedicine was formed by chelation between a drug peptide amphiphile Rh-GFFYERGD (Rh represents Rhein, 1,8-dihydroxy-3-carboxy anthraquinonea) and cisplatinum (Pt). A single molecule of the drug peptide amphiphile could chelate up to 8 equiv. of cisplatinum in the resulting nanofibers. The nanofibers with a 1 : 4 ratio of Rh-GFFYERGD to cisplatinum demonstrated remarkable cellular uptake, and more significantly, superior nuclear accumulation properties. Additionally, the nanofibers could also bind to the DNA molecule more efficiently than those formed by the drug peptide amphiphile. Thus the nanofibers exhibited excellent anticancer properties both in vitro and in vivo. We envision a significant therapeutic potential of the dual anticancer drug-based nanomedicine with cisplatinum in cancer.
中文翻译:

顺铂诱导的肽自组装构建的基于双抗癌药物的纳米药物的核传递。
核传递抗癌药,特别是双重补充抗癌药,可以显着提高化疗效果。但是,成功的例子很少。我们报告了一种新型的双重抗癌药物为基础的具有核积累特性的纳米药物。通过在药物肽两亲物Rh-GFFYERGD(Rh代表大黄酸,1,8-二羟基-3-羧基蒽醌)和顺铂(Pt)之间螯合形成纳米药物。药物肽两亲物的单个分子可以螯合多达8当量。纳米纤维中的顺铂含量 Rh-GFFYERGD与顺铂之比为1:4的纳米纤维表现出显着的细胞摄取,更重要的是,具有优越的核积累特性。另外,与由药物肽两亲物形成的纳米纤维相比,纳米纤维还可以更有效地与DNA分子结合。因此,纳米纤维均显示出优异的抗癌性能。体外和体内。我们设想了基于双重抗癌药物的纳米药物与顺铂在癌症中的重大治疗潜力。
更新日期:2020-07-23
中文翻译:

顺铂诱导的肽自组装构建的基于双抗癌药物的纳米药物的核传递。
核传递抗癌药,特别是双重补充抗癌药,可以显着提高化疗效果。但是,成功的例子很少。我们报告了一种新型的双重抗癌药物为基础的具有核积累特性的纳米药物。通过在药物肽两亲物Rh-GFFYERGD(Rh代表大黄酸,1,8-二羟基-3-羧基蒽醌)和顺铂(Pt)之间螯合形成纳米药物。药物肽两亲物的单个分子可以螯合多达8当量。纳米纤维中的顺铂含量 Rh-GFFYERGD与顺铂之比为1:4的纳米纤维表现出显着的细胞摄取,更重要的是,具有优越的核积累特性。另外,与由药物肽两亲物形成的纳米纤维相比,纳米纤维还可以更有效地与DNA分子结合。因此,纳米纤维均显示出优异的抗癌性能。体外和体内。我们设想了基于双重抗癌药物的纳米药物与顺铂在癌症中的重大治疗潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号