当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enriched pseudocapacitive lithium storage in electrochemically activated carbonaceous vanadium(IV, V) oxide hydrate
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0ta04191b Joseph F. S. Fernando 1, 2, 3, 4, 5 , Dumindu P. Siriwardena 1, 2, 3, 4, 5 , Konstantin L. Firestein 1, 2, 3, 4, 5 , Chao Zhang 1, 2, 3, 4, 5 , Joel E. von Treifeldt 1, 2, 3, 4, 5 , Courtney-Elyce M. Lewis 1, 2, 3, 4, 5 , Tony Wang 1, 2, 3, 4, 6 , Deepak P. Dubal 1, 2, 3, 4, 5 , Dmitri V. Golberg 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0ta04191b Joseph F. S. Fernando 1, 2, 3, 4, 5 , Dumindu P. Siriwardena 1, 2, 3, 4, 5 , Konstantin L. Firestein 1, 2, 3, 4, 5 , Chao Zhang 1, 2, 3, 4, 5 , Joel E. von Treifeldt 1, 2, 3, 4, 5 , Courtney-Elyce M. Lewis 1, 2, 3, 4, 5 , Tony Wang 1, 2, 3, 4, 6 , Deepak P. Dubal 1, 2, 3, 4, 5 , Dmitri V. Golberg 1, 2, 3, 4, 5
Affiliation
|
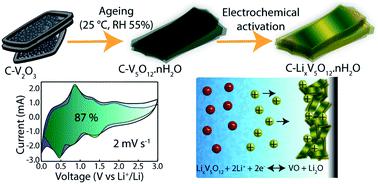
Hydrated vanadium oxides (HVOs) are promising (extrinsic) pseudocapacitive materials that extend the possibility to optimize electrochemical performance through fine-tuning their nanoscale porosity, structural disorder and conventional composition. These materials demonstrate the features of batteries (i.e. high energy density) and supercapacitors (fast power output). However, most pseudocapacitive oxides are characterized by poor conductivity, which leads to high electrode resistance. Herein, an amorphous-like carbon-integrated vanadium oxide hydrate (V5O12·0.4H2O, CHVO) is engineered using a straightforward solvothermal approach, with improved conductivity, rich pore architecture and readily available nanoscale redox-active sites. The CHVO material shows superior performance as an anode in Li-ion batteries with a specific capacity of 1175 mA h g−1 at 50 mA g−1 in the 150th cycle, which was maintained to 525 mA h g−1 over 600 cycles at 1000 mA g−1. The half-cell can be charged to 403 mA h g−1 (1451 C g−1) at 4000 mA g−1 in ∼6 min. The cyclic voltammetry analysis shows that CHVO undergoes an electrochemical activation by forming a stable lithium vanadium oxide in the initial cycles. The research on electrochemical de(lithiation) mechanism of HVOs at low potentials (<1 V) is largely unexplored. In this context, real time measurements using in situ Transmission Electron Microscopy are provided to underpin the Li-ion transport process in CHVO electrodes. It is revealed that the carbon framework and peculiar structure of CHVO effectively buffer large volume expansions upon (de)lithiation. In addition, ex situ X-ray photoelectron spectroscopy and X-ray Diffraction measurements were collectively utilized to uncover a conversion-type reaction mechanism.
中文翻译:

电化学活性碳钒酸钒(IV,V)水合物中富集的假电容锂存储
水合氧化钒(HVOs)是有前途的(外部)假电容材料,可通过微调其纳米级孔隙率,结构紊乱和常规组成来扩展优化电化学性能的可能性。这些材料展示了电池(即高能量密度)和超级电容器(快速输出功率)的功能。然而,大多数伪电容性氧化物的特征在于导电性差,这导致高电极电阻。在此,为无定形的碳结合钒氧化物水合物(V 5 O 12 ·0.4H 2O,CHVO)是使用直接的溶剂热方法设计的,具有改进的电导率,丰富的孔结构和易于获得的纳米级氧化还原活性位点。CHVO材料在锂离子电池中表现出优异的阳极性能,在第150个循环中在50 mA g -1下的比容量为1175 mA hg -1,在1000个循环中的600个循环中保持为525 mA hg -1 mA g -1。半电池可以在4000 mA g -1下充电至403 mA hg -1(1451 C g -1)在约6分钟内 循环伏安法分析表明,CHVO在初始循环中通过形成稳定的锂钒氧化物进行了电化学活化。在低电势(<1 V)下,HVOs的电化学脱锂(锂化)机理的研究尚待探索。在这种情况下,提供了使用原位透射电子显微镜的实时测量,以支持CHVO电极中锂离子的传输过程。揭示了CHVO的碳骨架和特殊结构有效地缓冲了(去)锂化时的大体积膨胀。此外,异位X射线光电子能谱和X射线衍射测量被共同用来揭示转化型反应机理。
更新日期:2020-07-07
中文翻译:

电化学活性碳钒酸钒(IV,V)水合物中富集的假电容锂存储
水合氧化钒(HVOs)是有前途的(外部)假电容材料,可通过微调其纳米级孔隙率,结构紊乱和常规组成来扩展优化电化学性能的可能性。这些材料展示了电池(即高能量密度)和超级电容器(快速输出功率)的功能。然而,大多数伪电容性氧化物的特征在于导电性差,这导致高电极电阻。在此,为无定形的碳结合钒氧化物水合物(V 5 O 12 ·0.4H 2O,CHVO)是使用直接的溶剂热方法设计的,具有改进的电导率,丰富的孔结构和易于获得的纳米级氧化还原活性位点。CHVO材料在锂离子电池中表现出优异的阳极性能,在第150个循环中在50 mA g -1下的比容量为1175 mA hg -1,在1000个循环中的600个循环中保持为525 mA hg -1 mA g -1。半电池可以在4000 mA g -1下充电至403 mA hg -1(1451 C g -1)在约6分钟内 循环伏安法分析表明,CHVO在初始循环中通过形成稳定的锂钒氧化物进行了电化学活化。在低电势(<1 V)下,HVOs的电化学脱锂(锂化)机理的研究尚待探索。在这种情况下,提供了使用原位透射电子显微镜的实时测量,以支持CHVO电极中锂离子的传输过程。揭示了CHVO的碳骨架和特殊结构有效地缓冲了(去)锂化时的大体积膨胀。此外,异位X射线光电子能谱和X射线衍射测量被共同用来揭示转化型反应机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号