当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Infrared Spectra of (Z)- and (E)-•C2H3C(CH3)I Radicals Produced upon Photodissociation of (Z)- and (E)-(CH2I)HC═C(CH3)I in Solid para-Hydrogen.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jpca.0c03987 Karolina Anna Haupa,Kuang-Po Chen,Yaw-Kuen Li,Yuan-Pern Lee
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jpca.0c03987 Karolina Anna Haupa,Kuang-Po Chen,Yaw-Kuen Li,Yuan-Pern Lee
|
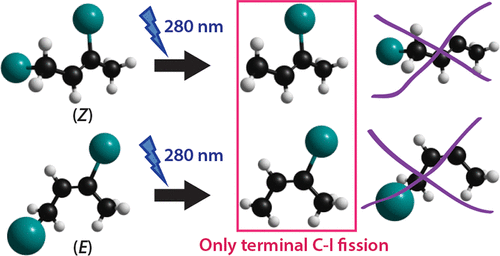
Ozonolysis of isoprene to produce Criegee intermediates such as methyl vinyl ketone oxide (MVKO), C2H3C(CH3)OO, is an important process in atmospheric chemistry. MVKO was recently produced and identified in laboratories after photolysis of a gaseous mixture of 1,3-diiodo-but-2-ene, (CH2I)HC═C(CH3)I, and O2, but the mechanism of its formation remains unexplored. We synthesized pure (Z)- and (E)-1,3-diiodo-but-2-ene and measured their distinct IR spectra. Upon irradiation at 280 nm of (Z)- and (E)-1,3-diiodo-but-2-ene in solid p-H2 at 3.3 K, the fission of the terminal C—I bond yields (Z)- and (E)-3-iodo-but-2-en-1-yl [•C2H3C(CH3)I] radicals, respectively. These radicals were characterized with infrared absorption lines at 2962.4, 1423.8, 1265.3, 1120.9/1127.0, 921.4/922.3, and 792.5/791.7 cm–1, and 16 additional weaker lines for (Z)-•C2H3C(CH3)I and 1405.2, 1208.2, 1106.0/1103.9, 934.2/933.4, and 785.1/784.9 cm–1 and five additional weaker ones for (E)-•C2H3C(CH3)I. The assignments were derived according to behavior on secondary photolysis and comparison of the vibrational wavenumbers and the IR intensities of observed lines with those calculated with the B2PLYP-D3/aug-cc-pVTZ-pp method. These observations confirmed that only the terminal I atom, not the central one, was photodissociated at 280 nm and, in solid p-H2, the excess energy after photodissociation induced no change in conformation. These new spectra of •C2H3C(CH3)I radicals can provide valuable information for the understanding of the mechanism of formation of Criegee intermediate MVKO from the source reaction of photolysis of (CH2I)HC═C(CH3)I in O2 in the laboratory.
中文翻译:

(Z)-和(E)-•C2H3C(CH3)I自由基的红外光谱,是由(Z)-和(E)-(CH2I)HC═C(CH3)I在固体对氢中光解离而产生的。
异戊二烯的臭氧分解以产生Criegee中间体,例如甲基乙烯基酮氧化物(MVKO),C 2 H 3 C(CH 3)OO是大气化学中的重要过程。MVKO是在将1,3-二碘丁-2-烯,(CH 2 I)HC═C(CH 3)I和O 2的气态混合物光解后在实验室中生产和鉴定的,但其机理是形成仍未探索。我们合成了纯的(Z)-和(E)-1,3-二碘代丁-2-烯,并测量了它们独特的IR光谱。固体p -H 2中的(Z)-和(E)-1,3-二碘代丁-2-烯在280 nm处辐照在3.3 K时,末端C-I键的裂变产生(Z)-和(E)-3-碘-丁-2-烯-1-基[ • C 2 H 3 C(CH 3)I]基团, 分别。这些自由基的特征是在2962.4、1423.8、1265.3、1120.9 / 1127.0、921.4 / 922.3和792.5 / 791.7 cm –1处具有红外吸收谱线,并且对于(Z)- • C 2 H 3 C(CH 3)I和1405.2、1208.2、1106.0 / 1103.9、934.2 / 933.4和785.1 / 784.9 cm –1,以及(E)- • C 2的另外五个较弱的H 3 C(CH 3)I。根据二次光解的行为以及将观察到的谱线的振动波数和IR强度与用B2PLYP-D3 / aug-cc-pVTZ-pp方法计算得到的谱线进行比较,得出分配。这些观察结果证实,仅末端I原子而不是中心原子在280nm处光解离,并且在固体p- H 2中,光解后的过量能量不引起构象变化。这些新的光谱• ç 2 ħ 3 C(CH 3I自由基可为实验室中O 2中(CH 2 I)HC═C(CH 3)I的光解反应中的克里基中间体MVKO的形成机理提供有价值的信息。
更新日期:2020-07-16
中文翻译:

(Z)-和(E)-•C2H3C(CH3)I自由基的红外光谱,是由(Z)-和(E)-(CH2I)HC═C(CH3)I在固体对氢中光解离而产生的。
异戊二烯的臭氧分解以产生Criegee中间体,例如甲基乙烯基酮氧化物(MVKO),C 2 H 3 C(CH 3)OO是大气化学中的重要过程。MVKO是在将1,3-二碘丁-2-烯,(CH 2 I)HC═C(CH 3)I和O 2的气态混合物光解后在实验室中生产和鉴定的,但其机理是形成仍未探索。我们合成了纯的(Z)-和(E)-1,3-二碘代丁-2-烯,并测量了它们独特的IR光谱。固体p -H 2中的(Z)-和(E)-1,3-二碘代丁-2-烯在280 nm处辐照在3.3 K时,末端C-I键的裂变产生(Z)-和(E)-3-碘-丁-2-烯-1-基[ • C 2 H 3 C(CH 3)I]基团, 分别。这些自由基的特征是在2962.4、1423.8、1265.3、1120.9 / 1127.0、921.4 / 922.3和792.5 / 791.7 cm –1处具有红外吸收谱线,并且对于(Z)- • C 2 H 3 C(CH 3)I和1405.2、1208.2、1106.0 / 1103.9、934.2 / 933.4和785.1 / 784.9 cm –1,以及(E)- • C 2的另外五个较弱的H 3 C(CH 3)I。根据二次光解的行为以及将观察到的谱线的振动波数和IR强度与用B2PLYP-D3 / aug-cc-pVTZ-pp方法计算得到的谱线进行比较,得出分配。这些观察结果证实,仅末端I原子而不是中心原子在280nm处光解离,并且在固体p- H 2中,光解后的过量能量不引起构象变化。这些新的光谱• ç 2 ħ 3 C(CH 3I自由基可为实验室中O 2中(CH 2 I)HC═C(CH 3)I的光解反应中的克里基中间体MVKO的形成机理提供有价值的信息。

























 京公网安备 11010802027423号
京公网安备 11010802027423号