当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Cyclopropanes via 1,3-Migration of Acyloxy Groups Triggered by Formation of α-Imino Rhodium Carbenes.
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.orglett.0c01764 Ze-Feng Xu 1, 2 , Jincheng Liu 1 , Xin Chang 1 , Tao Chen 1 , Huaping Xu 1 , Shengguo Duan 1 , Chuan-Ying Li 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.orglett.0c01764 Ze-Feng Xu 1, 2 , Jincheng Liu 1 , Xin Chang 1 , Tao Chen 1 , Huaping Xu 1 , Shengguo Duan 1 , Chuan-Ying Li 1
Affiliation
|
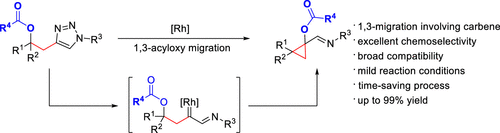
A novel and highly efficient synthetic approach to cyclopropanes was realized via 1,3-migration of acyloxy groups triggered by α-imino rhodium carbenes. Excellent chemoselectivity ensured broad compatibility of common functional groups. Merits such as readily available substrates, mild reaction conditions, and time-saving processes qualified this transformation as an attractive alternative strategy to synthesize multifunctionalized cyclopropanes. Primary investigations and discussion on the mechanism are presented.
中文翻译:

通过α-亚氨基铑卡宾酸酯的形成引发的乙酰氧基的1,3-迁移来合成环丙烷。
通过α-亚氨基铑卡宾烯引发的酰氧基的1,3-迁移,实现了一种新型的高效合成环丙烷的方法。出色的化学选择性确保了常见官能团的广泛相容性。诸如易于获得的底物,温和的反应条件和省时的工艺等优点使这种转化成为合成多功能环丙烷的有吸引力的替代策略。介绍了对该机制的初步研究和讨论。
更新日期:2020-07-02
中文翻译:

通过α-亚氨基铑卡宾酸酯的形成引发的乙酰氧基的1,3-迁移来合成环丙烷。
通过α-亚氨基铑卡宾烯引发的酰氧基的1,3-迁移,实现了一种新型的高效合成环丙烷的方法。出色的化学选择性确保了常见官能团的广泛相容性。诸如易于获得的底物,温和的反应条件和省时的工艺等优点使这种转化成为合成多功能环丙烷的有吸引力的替代策略。介绍了对该机制的初步研究和讨论。


























 京公网安备 11010802027423号
京公网安备 11010802027423号