当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility and Thermodynamic Model Correlation of Zonisamide in Different Pure Solvents from T = (273.15 to 313.15) K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.jced.0c00249 Guoquan Zhou 1 , Ke Chen 2 , Zehui Yang 1 , Danfeng Shao 1 , Huajun Fan 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.jced.0c00249 Guoquan Zhou 1 , Ke Chen 2 , Zehui Yang 1 , Danfeng Shao 1 , Huajun Fan 3
Affiliation
|
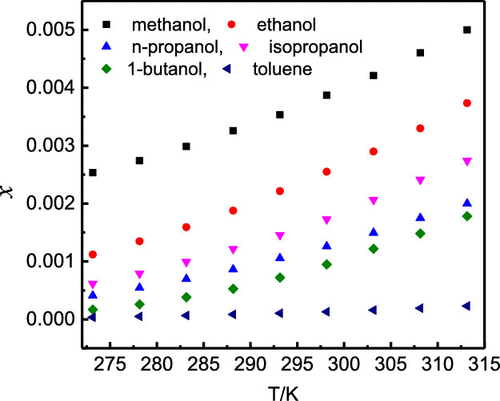
Zonisamide is moderately soluble in water with a reported solubility of 0.8 mg·mol–1 (6.78 × 10–5 in mole fraction). The objective is to study the solubility of zonisamide in methanol, ethanol, n-propanol, isopropanol, 1-butanol, acetonitrile, ethyl acetate, toluene, 2-butanone, cyclohexanone, 1,4-dioxane, and N,N-dimethylformamide (DMF) at the temperature range from 273.15 to 313.15 K, which was measured using the isothermal dissolution equilibrium method under the pressure of 101.2 kPa. The largest solubility value was found in DMF followed by 1,4-dioxane > 2-butanone > cyclohexanone > acetonitrile > ethyl acetate > methanol > ethanol > isopropanol > n-propanol > 1-butanol > toluene. It could be found that the solubility in organic solvent was much higher than that in water. The solubility result in different solvents was correlated by the modified Apelblat equation and λh equation nonrandom two-liquid (NRTL) model. In the modified Apelblat equation, the maximum data is 1.57%. However, the largest relative average deviation (RAD) of λh equation and NRTL model was obtained in 1-butanol and was more than 5%. All RAD values in the modified Apelblat equation are smaller than those in λh equation and NRTL model. Therefore, the modified Apelblat equation is more suitable to correlate the solubility data of zonisamide in all selected solvents.
中文翻译:

T =(273.15至313.15)K在不同纯溶剂中唑尼沙胺的溶解度和热力学模型相关性
唑尼沙胺在水中的溶解度中等,据报道溶解度为0.8 mg·mol –1(摩尔分数为6.78×10 –5)。目的是研究唑尼沙胺在甲醇,乙醇,正丙醇,异丙醇,1-丁醇,乙腈,乙酸乙酯,甲苯,2-丁酮,环己酮,1,4-二恶烷和N,N-二甲基甲酰胺中的溶解度( (DMF)在273.15至313.15 K的温度范围内,使用等温溶解平衡方法在101.2 kPa的压力下测量。在DMF中发现最大的溶解度值,其次是1,4-二恶烷> 2-丁酮>环己酮>乙腈>乙酸乙酯>甲醇>乙醇>异丙醇> n-丙醇> 1-丁醇>甲苯。可以发现在有机溶剂中的溶解度远高于在水中的溶解度。在不同溶剂中的溶解性结果用改良Apelblat方程和λ相关ħ方程非随机两液体(NRTL)模型。在修改后的Apelblat方程中,最大数据为1.57%。然而,λ最大相对平均偏差(RAD)ħ在1-丁醇,得到方程和NRTL模型和为5%以上。改性Apelblat方程中的所有RAD值比在λ较小ħ方程和NRTL模型。因此,修改后的Apelblat方程更适合关联zonisamide在所有选定溶剂中的溶解度数据。
更新日期:2020-07-09
中文翻译:

T =(273.15至313.15)K在不同纯溶剂中唑尼沙胺的溶解度和热力学模型相关性
唑尼沙胺在水中的溶解度中等,据报道溶解度为0.8 mg·mol –1(摩尔分数为6.78×10 –5)。目的是研究唑尼沙胺在甲醇,乙醇,正丙醇,异丙醇,1-丁醇,乙腈,乙酸乙酯,甲苯,2-丁酮,环己酮,1,4-二恶烷和N,N-二甲基甲酰胺中的溶解度( (DMF)在273.15至313.15 K的温度范围内,使用等温溶解平衡方法在101.2 kPa的压力下测量。在DMF中发现最大的溶解度值,其次是1,4-二恶烷> 2-丁酮>环己酮>乙腈>乙酸乙酯>甲醇>乙醇>异丙醇> n-丙醇> 1-丁醇>甲苯。可以发现在有机溶剂中的溶解度远高于在水中的溶解度。在不同溶剂中的溶解性结果用改良Apelblat方程和λ相关ħ方程非随机两液体(NRTL)模型。在修改后的Apelblat方程中,最大数据为1.57%。然而,λ最大相对平均偏差(RAD)ħ在1-丁醇,得到方程和NRTL模型和为5%以上。改性Apelblat方程中的所有RAD值比在λ较小ħ方程和NRTL模型。因此,修改后的Apelblat方程更适合关联zonisamide在所有选定溶剂中的溶解度数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号