当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interaction between PbO and the KCl–PbCl2–PbO System for Lead Electrochemical Refining
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jced.0c00080 Pavel Arkhipov 1 , Yurii Zaikov 1 , Aleksandr Katayev 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jced.0c00080 Pavel Arkhipov 1 , Yurii Zaikov 1 , Aleksandr Katayev 1
Affiliation
|
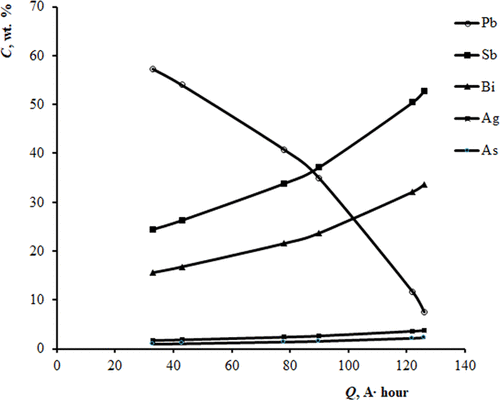
A KCl–PbCl2 (50.0–50.0 mol %) molten system was used as an electrolyte for secondary lead refining. As a result, pure lead and an anode product containing 52.81 mol % Sb, 33.66 mol % Bi, 7.54 mol % Pb, 3.74 mol % Ag, and 2.24 mol % As were obtained. Long-term electrochemical refining was found to be possible in the KCl–PbCl2 electrolyte. Thermal analysis was applied to determine liquidus temperatures of the KCl–PbCl2 (50.0–50.0 mol %) mixture with the PbO concentration ranging from 0 to 20 mol %. The temperature dependence of the PbO solubility in the KCl–PbCl2 (50.0–50.0 mol %) melt was studied, and the thermodynamic parameters of the PbO solubility were calculated. Electrical conductivities and densities of oxy-chloride melts were measured.
中文翻译:

PbO与KCl–PbCl 2 –PbO系统之间的相互作用用于铅电化学精炼
使用KCl-PbCl 2(50.0-50.0 mol%)熔融体系作为二次精炼的电解质。结果,获得了纯铅和包含52.81 mol%Sb,33.66 mol%Bi,7.54 mol%Pb,3.74 mol%Ag和2.24 mol%As的阳极产物。发现在KCl–PbCl 2电解质中可以进行长期的电化学精制。通过热分析确定KCl–PbCl 2(50.0–50.0 mol%)混合物的液相线温度,PbO浓度为0至20 mol%。PbO在KCl–PbCl 2中的溶解度与温度的关系研究了(50.0-50.0 mol%)熔体,并计算了PbO溶解度的热力学参数。测量了氯氧化物熔体的电导率和密度。
更新日期:2020-07-09
中文翻译:

PbO与KCl–PbCl 2 –PbO系统之间的相互作用用于铅电化学精炼
使用KCl-PbCl 2(50.0-50.0 mol%)熔融体系作为二次精炼的电解质。结果,获得了纯铅和包含52.81 mol%Sb,33.66 mol%Bi,7.54 mol%Pb,3.74 mol%Ag和2.24 mol%As的阳极产物。发现在KCl–PbCl 2电解质中可以进行长期的电化学精制。通过热分析确定KCl–PbCl 2(50.0–50.0 mol%)混合物的液相线温度,PbO浓度为0至20 mol%。PbO在KCl–PbCl 2中的溶解度与温度的关系研究了(50.0-50.0 mol%)熔体,并计算了PbO溶解度的热力学参数。测量了氯氧化物熔体的电导率和密度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号