Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.saa.2020.118619 Sachie Kudo 1 , Satoru Nakashima 2
|
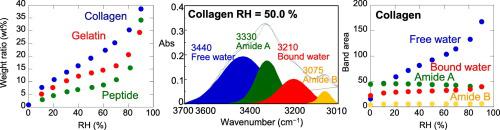
In this study, water retention properties of triple helix collagen, gelatin (separated single chains) and peptide (broken peptide fragments) were studied by using IR micro-spectroscopy equipped with a relative humidity (RH) control system and quartz crystal microbalance (QCM). Adsorbed water ratios (wt%) are found to be in the order of collagen, gelatin and peptide (at about RH = 60%, 22 wt% for collagen, 14 wt% for gelatin and 9 wt% for peptide). Free water molecules with longer H bonds are the major adsorbed water species for collagen, gelatin and peptide. IR band shifts and changes in normalized band areas of functional groups are generally larger for collagen than gelatin and peptide, indicating larger interactions of water molecules with functional groups such as aliphatic CH2, CH3, amides, COO− and C–O for collagen. Relations between normalized band areas show that water molecules are interacting with aliphatic CH species and C–O bonds of collagen. Since the fibril structures of collagen triple helices are reported to be cross-linked by sugars, water molecules can be attracted to polar C–O bonds of sugars linking collagen triple helices in fibrils and they are interacting with adjacent aliphatic CH side chains on the surface of fibrils.
中文翻译:

通过IR / QCM / RH系统研究的胶原蛋白,明胶和肽的保水能力。
在这项研究中,通过配备相对湿度(RH)控制系统和石英晶体微量天平(QCM)的红外显微技术研究了三螺旋胶原蛋白,明胶(分离的单链)和肽(断裂的肽片段)的保水性能。 。发现吸附水的比率(wt%)为胶原蛋白,明胶和肽的顺序(在约RH = 60%,胶原蛋白为22wt%,明胶为14wt%和肽为9wt%)。具有较长H键的自由水分子是胶原,明胶和肽的主要吸附水种类。对于胶原蛋白,IR谱带移动和官能团归一化谱带区域的变化通常比明胶和肽更大,这表明水分子与脂肪族CH 2,CH 3等官能团的相互作用更大,酰胺,COO -和C-O为胶原。归一化带面积之间的关系表明,水分子与脂肪族CH物种和胶原的C–O键相互作用。由于据报道胶原三螺旋的原纤维结构被糖交联,水分子可被糖连接到原纤维中胶原三螺旋的糖的极性C–O键吸引,并且糖与表面上相邻的脂肪族CH侧链相互作用原纤维。


























 京公网安备 11010802027423号
京公网安备 11010802027423号