Immunity ( IF 32.4 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.immuni.2020.06.008 Alicia Bellomo 1 , Isabelle Mondor 1 , Lionel Spinelli 1 , Marine Lagueyrie 1 , Benjamin J Stewart 2 , Nicolas Brouilly 3 , Bernard Malissen 1 , Menna R Clatworthy 2 , Marc Bajénoff 1
|
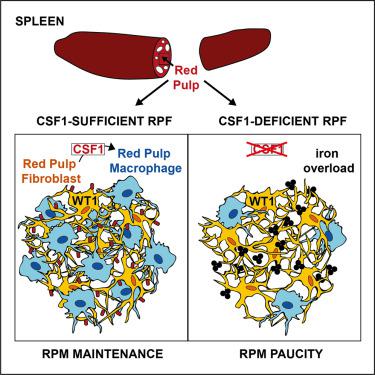
Located within red pulp cords, splenic red pulp macrophages (RPMs) are constantly exposed to the blood flow, clearing senescent red blood cells (RBCs) and recycling iron from hemoglobin. Here, we studied the mechanisms underlying RPM homeostasis, focusing on the involvement of stromal cells as these cells perform anchoring and nurturing macrophage niche functions in lymph nodes and liver. Microscopy revealed that RPMs are embedded in a reticular meshwork of red pulp fibroblasts characterized by the expression of the transcription factor Wilms’ Tumor 1 (WT1) and colony stimulating factor 1 (CSF1). Conditional deletion of Csf1 in WT1+ red pulp fibroblasts, but not white pulp fibroblasts, drastically altered the RPM network without altering circulating CSF1 levels. Upon RPM depletion, red pulp fibroblasts transiently produced the monocyte chemoattractants CCL2 and CCL7, thereby contributing to the replenishment of the RPM network. Thus, red pulp fibroblasts anchor and nurture RPM, a function likely conserved in humans.
中文翻译:

表达转录因子 WT1 的网状成纤维细胞定义了一个维持和补充脾脏红髓巨噬细胞的基质生态位。
位于红髓索内的脾脏红髓巨噬细胞 (RPM) 不断暴露在血流中,清除衰老的红细胞 (RBC) 并从血红蛋白中回收铁。在这里,我们研究了 RPM 稳态的潜在机制,重点关注基质细胞的参与,因为这些细胞在淋巴结和肝脏中执行锚定和培育巨噬细胞生态位功能。显微镜检查显示 RPM 嵌入红髓成纤维细胞的网状网络中,其特征在于转录因子 Wilms 肿瘤 1 (WT1) 和集落刺激因子 1 (CSF1) 的表达。WT1 +中Csf1的条件删除红髓成纤维细胞,而不是白髓成纤维细胞,在不改变循环 CSF1 水平的情况下显着改变了 RPM 网络。RPM 耗尽后,红髓成纤维细胞瞬时产生单核细胞趋化因子 CCL2 和 CCL7,从而有助于 RPM 网络的补充。因此,红髓成纤维细胞锚定和培养 RPM,这种功能可能在人类中是保守的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号