Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.bioorg.2020.104033 Michał Załuski 1 , Jakub Schabikowski 1 , Piotr Jaśko 2 , Adrian Bryła 2 , Agnieszka Olejarz-Maciej 1 , Maria Kaleta 1 , Monika Głuch-Lutwin 3 , Andreas Brockmann 4 , Sonja Hinz 4 , Małgorzata Zygmunt 2 , Kamil Kuder 1 , Gniewomir Latacz 1 , Christin Vielmuth 4 , Christa E Müller 4 , Katarzyna Kieć-Kononowicz 1
|
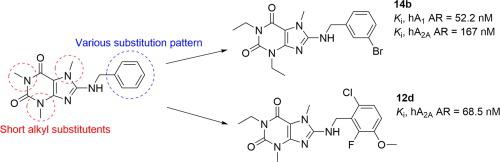
A library of 34 novel compounds based on a xanthine scaffold was explored in biological studies for interaction with adenosine receptors (ARs). Structural modifications of the xanthine core were introduced in the 8-position (benzylamino and benzyloxy substitution) as well as at N1, N3, and N7 (small alkyl residues), thereby improving affinity and selectivity for the A2A AR. The compounds were characterized by radioligand binding assays, and our study resulted in the development of the potent A2A AR ligands including 8-((6-chloro-2-fluoro-3-methoxybenzyl)amino)-1-ethyl-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (12d; Ki human A2AAR: 68.5 nM) and 8-((2-chlorobenzyl)amino)-1-ethyl-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione (12h; Ki human A2AAR: 71.1 nM). Moreover, dual A1/A2AAR ligands were identified in the group of 1,3-diethyl-7-methylxanthine derivatives. Compound 14b displayed Ki values of 52.2 nM for the A1AR and 167 nM for the A2AAR. Selected A2AAR ligands were further evaluated as inactive for inhibition of monoamine oxidase A, B and isoforms of phosphodiesterase-4B1, -10A, which represent classical targets for xanthine derivatives. Therefore, the developed 8-benzylaminoxanthine scaffold seems to be highly selective for AR activity and relevant for potent and selective A2A ligands. Compound 12d with high selectivity for ARs, especially for the A2AAR subtype, evaluated in animal models of inflammation has shown anti-inflammatory activity. Investigated compounds were found to display high selectivity and may therefore be of high interest for further development as drugs for treating cancer or neurodegenerative diseases.
中文翻译:

作用于腺苷A2A受体的选择性配体的8-Benzylaminoxanthine支架变异。设计,合成和生物学评估。
在生物学研究中探索了基于黄嘌呤骨架的34种新化合物的文库,用于与腺苷受体(ARs)相互作用。黄嘌呤核的结构修饰被引入8位(苄氨基和苄氧基取代)以及N 1,N 3和N 7(小的烷基残基),从而提高了对A 2A AR的亲和力和选择性。通过放射性配体结合试验对化合物进行了表征,我们的研究导致开发了有效的A 2A AR配体,其中包括8-((6-氯-2-氟-3-甲氧基苄基)氨基)-1-乙基-3,7 -二甲基-3,7-二氢-1 H-嘌呤-2,6-二酮(12d ; K i人A 2A AR:68.5 nM)和8-((2-氯苄基)氨基)-1-乙基-3,7-二甲基-3,7-二氢-1 H-嘌呤-2,6-二酮(12 h ; K i人A 2A AR:71.1 nM)。此外,在1,3-二乙基-7-甲基黄嘌呤衍生物的组中鉴定出双A 1 / A 2A AR配体。化合物14b的显示ķ我为A值52.2 NM的1 AR和167 nM的用于A 2A AR。选择A 2A进一步评估了AR配体对于抑制单胺氧化酶A,B和磷酸二酯酶-4B1,-10A的同工型是无效的,它们代表了黄嘌呤衍生物的经典靶标。因此,开发的8-苄基氨基黄嘌呤支架似乎对AR活性具有高度选择性,并与有效和选择性的A 2A配体有关。在炎症动物模型中评估的对ARs特别是对A 2A AR亚型具有高选择性的化合物12d已显示出抗炎活性。发现所研究的化合物显示出高选择性,因此作为治疗癌症或神经退行性疾病的药物对于进一步开发可能具有很高的兴趣。



























 京公网安备 11010802027423号
京公网安备 11010802027423号