Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.bioorg.2020.104031 Angel H Romero 1 , Felipe Sojo 2 , Francisco Arvelo 2 , Christian Calderón 3 , Alvaro Morales 4 , Simón E López 5
|
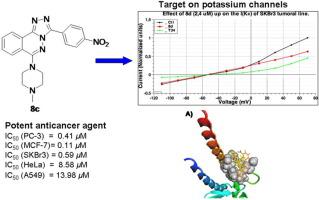
A series of six 3-aryl-6-(N-methylpiperazin)-1,2,4-triazolo[3,4-a]phthalazines were prepared through a facile and efficient one-pot copper-catalyzed procedure from 4-chloro-1-phthalazinyl-arylhydrazones with relatively good yields (62–83%). The one-pot copper-catalytic procedure consists of two simultaneous reactions: (i) a direct intramolecular dehydrogentaive cyclization between ylidenic carbon and adjacent pyrazine nitrogen to form 1,2,4-triazolo ring and, (ii) a direct N-amination on carbon-chlorine bond. Then, an in vitro anticancer evaluation was performed for the synthesized compounds against five selected human cancer cells (A549, MCF-7, SKBr3, PC-3 and HeLa). The nitro-derivatives were significantly more active against cancer strains than against the rest of tested compounds. Specifically, compound 8d was identified as the most promising anticancer agent with significant biological responses and low relative toxicities on human dermis fibroblast. The cytotoxic effect of compound 8d was more significant on PC3, MCF-7 and SKBr3 cancer cells with low-micromolar IC50 value ranging from 0.11 to 0.59 μM, superior to Adriamycin drug. Mechanistic experimental and theoretical studies demonstrated that compounds 8d act as a K+ channel inhibitor in cancer models. Further molecular docking studies suggest that the EGFR Tyrosine Kinase enzyme may be a potential target for the most active 3-aryl-6-(N-methylpiperazin)-1,2,4-triazolo[3,4-a]phthalazines.
中文翻译:

新型的3-硝基芳基-6-(N-甲基)哌嗪-1,2,4-三唑并[3,4-a]酞嗪类化合物对电压门控性K +通道的抗癌潜力:铜催化的4-氯一锅合成-1-酞嗪基-芳基azo。
通过简便有效的一锅铜催化程序,从4-氯代-3-氯苯甲酸制备了一系列六个3-芳基-6-(N-甲基哌嗪)-1,2,4-三唑并[3,4- a ]酞嗪。 1-酞嗪基-芳基hydr具有相对较好的产率(62–83%)。一锅法铜催化程序包括两个同时发生的反应:(i)分子内碳与相邻的吡嗪氮之间的直接分子内脱氢热化环化反应,形成1,2,4-三唑环,(ii)在反应中进行直接N胺化碳-氯键。然后,在体外对合成的化合物针对五种选定的人类癌细胞(A549,MCF-7,SKBr3,PC-3和HeLa)进行了抗癌评估。硝基衍生物对癌症菌株的活性比对其余测试化合物的活性高得多。具体而言,化合物8d被确定为最有前途的抗癌药,对人真皮成纤维细胞具有显着的生物学反应且相对毒性较低。化合物8d对PC3,MCF-7和SKBr3癌细胞的细胞毒性作用更为显着,其低微摩尔IC 50值为0.11至0.59μM,优于阿霉素药物。机理实验和理论研究表明,化合物8d可作为K +癌症模型中的通道抑制剂。进一步的分子对接研究表明,EGFR酪氨酸激酶可能是最具活性的3-芳基-6-(N-甲基哌嗪)-1,2,4-三唑并[3,4- a ]酞嗪的潜在靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号