Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Terbium-to-quantum dot Förster resonance energy transfer for homogeneous and sensitive detection of histone methyltransferase activity.
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0nr03383a Tooba Hallaj 1 , Mohammad Amjadi 2 , Xue Qiu 3 , Kimihiro Susumu 4 , Igor L Medintz 5 , Niko Hildebrandt 6
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0nr03383a Tooba Hallaj 1 , Mohammad Amjadi 2 , Xue Qiu 3 , Kimihiro Susumu 4 , Igor L Medintz 5 , Niko Hildebrandt 6
Affiliation
|
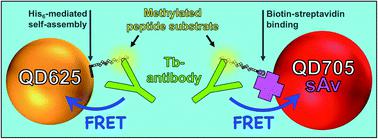
The development of rapid, simple, and versatile biosensors for monitoring the activity of histone modifying enzymes (HMEs) is needed for the improvement of diagnostic assays, screening of HME inhibitors, and a better understanding of HME kinetics in different environments. Nanoparticles can play an important role in this regard by improving or complementing currently available enzyme detection technologies. Here, we present the development and application of a homogeneous methyltransferase (SET7/9) assay based on time-gated Förster resonance energy transfer (TG-FRET) between terbium complexes (Tb) and luminescent semiconductor quantum dots (QDs). Specific binding of a Tb-antibody conjugate to a SET7/9-methylated Lys4 on a histone H3(1–21) peptide substrate attached to the QD surface resulted in efficient FRET and provided the mechanism for monitoring the SET7/9 activity. Two common peptide-QD attachment strategies (biotin–streptavidin and polyhistidine-mediated self-assembly), two different QD colors (625 and 705 nm), and enzyme sensing with post- or pre-assembled QD–peptide conjugates demonstrated the broad applicability of this assay design. Limits of detection in the low picomolar concentration range, high selectivity tested against non-specific antibodies, enzymes, and co-factors, determination of the inhibition constants of the SET7/9 inhibitors SAH and (R)-PFI-2, and analysis of the co-factor (SAM) concentration-dependent enzyme kinetics of SET7/9 which followed the Michaelis–Menten model highlighted the excellent performance of this TG-FRET HME activity assay.
中文翻译:

homogeneous到量子点的Förster共振能量转移,可对组蛋白甲基转移酶活性进行均质和灵敏检测。
需要开发快速,简单且通用的生物传感器来监测组蛋白修饰酶(HME)的活性,以改善诊断检测,筛选HME抑制剂以及更好地了解不同环境中HME动力学。在这方面,纳米颗粒可以通过改善或补充目前可用的酶检测技术来发挥重要作用。在这里,我们介绍基于and配合物(Tb)与发光半导体量子点(QD)之间的时间门控Förster共振能量转移(TG-FRET)的均相甲基转移酶(SET7 / 9)分析方法的开发和应用。Tb抗体偶联物与附着在QD表面的组蛋白H3(1-21)肽底物上的SET7 / 9-甲基化Lys4的特异性结合导致有效的FRET,并提供了监测SET7 / 9活性的机制。两种常见的肽-QD附着策略(生物素-链霉亲和素和多组氨酸介导的自组装),两种不同的QD颜色(625和705 nm)以及使用后或预组装的QD-肽结合物进行酶感测显示了广泛的适用性。此分析设计。在低皮摩尔浓度范围内的检测极限,针对非特异性抗体,酶和辅因子的高选择性,SET7 / 9抑制剂SAH和(R)-PFI-2的抑制常数的测定,
更新日期:2020-07-02
中文翻译:

homogeneous到量子点的Förster共振能量转移,可对组蛋白甲基转移酶活性进行均质和灵敏检测。
需要开发快速,简单且通用的生物传感器来监测组蛋白修饰酶(HME)的活性,以改善诊断检测,筛选HME抑制剂以及更好地了解不同环境中HME动力学。在这方面,纳米颗粒可以通过改善或补充目前可用的酶检测技术来发挥重要作用。在这里,我们介绍基于and配合物(Tb)与发光半导体量子点(QD)之间的时间门控Förster共振能量转移(TG-FRET)的均相甲基转移酶(SET7 / 9)分析方法的开发和应用。Tb抗体偶联物与附着在QD表面的组蛋白H3(1-21)肽底物上的SET7 / 9-甲基化Lys4的特异性结合导致有效的FRET,并提供了监测SET7 / 9活性的机制。两种常见的肽-QD附着策略(生物素-链霉亲和素和多组氨酸介导的自组装),两种不同的QD颜色(625和705 nm)以及使用后或预组装的QD-肽结合物进行酶感测显示了广泛的适用性。此分析设计。在低皮摩尔浓度范围内的检测极限,针对非特异性抗体,酶和辅因子的高选择性,SET7 / 9抑制剂SAH和(R)-PFI-2的抑制常数的测定,



























 京公网安备 11010802027423号
京公网安备 11010802027423号