当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Remarkably improved oxygen evolution reaction activity of cobalt oxides by an Fe ion solution immersion process
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0qi00385a Shencheng Pan 1, 2, 3, 4 , Xin Mao 5, 6, 7, 8, 9 , Juan Yu 1, 2, 3, 4 , Lin Hao 10, 11, 12, 13 , Aijun Du 5, 6, 7, 8, 9 , Bing Li 1, 2, 3, 4, 10
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0qi00385a Shencheng Pan 1, 2, 3, 4 , Xin Mao 5, 6, 7, 8, 9 , Juan Yu 1, 2, 3, 4 , Lin Hao 10, 11, 12, 13 , Aijun Du 5, 6, 7, 8, 9 , Bing Li 1, 2, 3, 4, 10
Affiliation
|
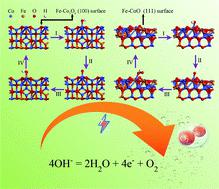
Cobalt oxides are economical and environmentally friendly electrocatalysts and they may be possible alternatives to noble metals for the oxygen evolution reaction. However, the most studied intrinsic cobalt oxides still need a high overpotential to overcome the current density threshold of oxygen evolution and are unstable for continuous reactions. Herein, we found that the catalytic activity of cobalt oxides improved significantly on soaking them in a weakly acidic iron–ion solution. The structure of the catalyst could be modified by the abovementioned treatment to reduce the overpotential, thus achieving improved catalytic efficiency for the oxygen evolution reaction. The formation of Fe-Co3O4 delivered a much lower overpotential of 280 mV at 10 mA cm−2 (with a small Tafel slope of 55 mV dec−1) compared with normal Co3O4 (409 mV) for the OER; additionally, Fe-CoO also exhibited a lower overpotential of 296 mV compared with normal CoO (361 mV). The activity of the Fe ion-treated cobalt oxides was promoted by a thousand CV scans, which might form an amorphous phase layer. Density functional theory (DFT) calculations also indicated that the theoretical overpotentials of Fe-Co3O4 and Fe-CoO were obviously lower than that of the untreated material. The treated cobalt oxides replaced cobalt atoms with a certain number of iron atoms, which dramatically changed the electronic structure and charge transfer rate.
中文翻译:

Fe离子溶液浸渍工艺显着提高了氧化钴的析氧反应活性
氧化钴是经济和环境友好的电催化剂,它们可能是氧气释放反应中贵金属的替代品。然而,研究最多的本征钴氧化物仍需要高的超电势才能克服氧释放的电流密度阈值,并且对于连续反应不稳定。在这里,我们发现将钴氧化物浸泡在弱酸性铁离子溶液中后,其催化活性得到了显着提高。通过上述处理可以改变催化剂的结构以减少过电势,从而实现改善的氧气释放反应的催化效率。Fe-Co 3 O 4的形成在10 mA cm -2时提供了低得多的280 mV的过电势(对于OER,与普通的Co 3 O 4(409 mV)相比,Tafel斜率为55 mV dec -1小);此外,Fe-CoO的过电位也比正常CoO(361 mV)低,为296 mV。Fe离子处理的钴氧化物的活性通过一千次CV扫描得到了增强,这可能形成非晶相层。密度泛函理论(DFT)计算还表明,Fe-Co 3 O 4和Fe-CoO的理论超电势明显低于未处理材料。经过处理的氧化钴用一定数量的铁原子代替了钴原子,从而极大地改变了电子结构和电荷转移速率。
更新日期:2020-06-18
中文翻译:

Fe离子溶液浸渍工艺显着提高了氧化钴的析氧反应活性
氧化钴是经济和环境友好的电催化剂,它们可能是氧气释放反应中贵金属的替代品。然而,研究最多的本征钴氧化物仍需要高的超电势才能克服氧释放的电流密度阈值,并且对于连续反应不稳定。在这里,我们发现将钴氧化物浸泡在弱酸性铁离子溶液中后,其催化活性得到了显着提高。通过上述处理可以改变催化剂的结构以减少过电势,从而实现改善的氧气释放反应的催化效率。Fe-Co 3 O 4的形成在10 mA cm -2时提供了低得多的280 mV的过电势(对于OER,与普通的Co 3 O 4(409 mV)相比,Tafel斜率为55 mV dec -1小);此外,Fe-CoO的过电位也比正常CoO(361 mV)低,为296 mV。Fe离子处理的钴氧化物的活性通过一千次CV扫描得到了增强,这可能形成非晶相层。密度泛函理论(DFT)计算还表明,Fe-Co 3 O 4和Fe-CoO的理论超电势明显低于未处理材料。经过处理的氧化钴用一定数量的铁原子代替了钴原子,从而极大地改变了电子结构和电荷转移速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号