当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water Transport Mechanisms of Poly(acrylic acid), Poly(vinyl alcohol), and Poly(ethylene glycol) in C-S-H Nanochannels: A Molecular Dynamics Study.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c03017 Jiao Yu 1 , Song Gao 1 , Dongshuai Hou 1 , Pan Wang 1 , Guoxing Sun 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c03017 Jiao Yu 1 , Song Gao 1 , Dongshuai Hou 1 , Pan Wang 1 , Guoxing Sun 2
Affiliation
|
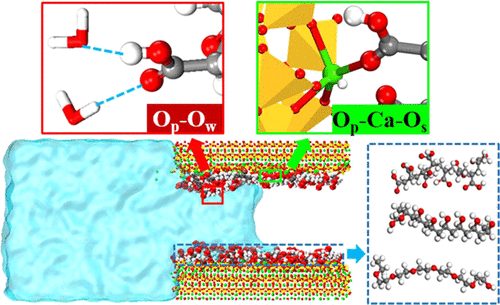
The transport properties of water molecules in nanochannels are critical to the durability of porous materials. In this article, molecular dynamics simulations are used to study the effects of poly(acrylic acid) (PAA), poly(vinyl alcohol) (PAA), and poly(ethylene glycol) (PEG) on the durability of modified cement-based materials. By establishing ideal composite nanopores, the absorption of water molecules in the channel is simulated. The results show that PEG has the best water-blocking effect under the same simulated conditions, followed by PVA, and PAA is the most unfavorable. This difference in the water-blocking effect can be explained by two factors. On the one hand, hydrophobic alkane groups in these polymers can inhibit water molecule transport. A large number of −COOH and −OH functional groups in PAA and PVA will form a complex H-bond network with the water molecules in the nanopore, dragging the water molecules forward, thereby speeding up the water molecule transmission to a certain extent. However, PEG, which mainly contains low-polar oxygen (C–O–C), has weak hydrogen bonding with water molecules, so the water-blocking effect is more obvious. On the other hand, the van der Waals interaction and the electrostatic interaction mainly derived from Op–Caw–Os can ensure the absorption of the polymer on the C–S–H surface during the transport process. The −COOH in PAA ensures its strongest absorption. But PVA and PEG will morphologically agglomerate during the water absorption, occupying pores and hindering the transport of water molecules.
中文翻译:

聚(丙烯酸),聚(乙烯醇)和聚(乙二醇)在CSH纳米通道中的输水机理:分子动力学研究。
水分子在纳米通道中的传输特性对于多孔材料的耐久性至关重要。在本文中,使用分子动力学模拟来研究聚丙烯酸(PAA),聚乙烯醇(PAA)和聚乙二醇(PEG)对改性水泥基材料耐久性的影响。通过建立理想的复合纳米孔,模拟了通道中水分子的吸收。结果表明,在相同的模拟条件下,PEG具有最佳的阻水效果,其次是PVA,而PAA最不利。阻水效果的这种差异可以由两个因素来解释。一方面,这些聚合物中的疏水性烷烃基团可以抑制水分子的运输。PAA和PVA中大量的-COOH和-OH官能团将与纳米孔中的水分子形成复杂的H键网络,将水分子向前拖动,从而在一定程度上加快了水分子的传输速度。但是,主要包含低极性氧(C–O–C)的PEG与水分子的氢键较弱,因此阻水作用更加明显。另一方面,范德华相互作用和静电相互作用主要来源于Op –Ca w –O s可以确保在运输过程中聚合物在C–S–H表面上的吸收。PAA中的-COOH可确保其最强的吸收性。但是PVA和PEG在吸水过程中会在形态上发生团聚,占据孔隙并阻碍水分子的运输。
更新日期:2020-07-16
中文翻译:

聚(丙烯酸),聚(乙烯醇)和聚(乙二醇)在CSH纳米通道中的输水机理:分子动力学研究。
水分子在纳米通道中的传输特性对于多孔材料的耐久性至关重要。在本文中,使用分子动力学模拟来研究聚丙烯酸(PAA),聚乙烯醇(PAA)和聚乙二醇(PEG)对改性水泥基材料耐久性的影响。通过建立理想的复合纳米孔,模拟了通道中水分子的吸收。结果表明,在相同的模拟条件下,PEG具有最佳的阻水效果,其次是PVA,而PAA最不利。阻水效果的这种差异可以由两个因素来解释。一方面,这些聚合物中的疏水性烷烃基团可以抑制水分子的运输。PAA和PVA中大量的-COOH和-OH官能团将与纳米孔中的水分子形成复杂的H键网络,将水分子向前拖动,从而在一定程度上加快了水分子的传输速度。但是,主要包含低极性氧(C–O–C)的PEG与水分子的氢键较弱,因此阻水作用更加明显。另一方面,范德华相互作用和静电相互作用主要来源于Op –Ca w –O s可以确保在运输过程中聚合物在C–S–H表面上的吸收。PAA中的-COOH可确保其最强的吸收性。但是PVA和PEG在吸水过程中会在形态上发生团聚,占据孔隙并阻碍水分子的运输。



























 京公网安备 11010802027423号
京公网安备 11010802027423号