当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
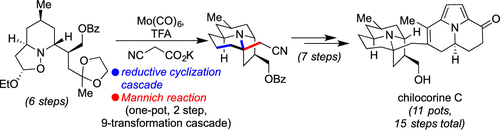
A Concise, Enantiospecific Total Synthesis of Chilocorine C Fueled by a Reductive Cyclization/Mannich Reaction Cascade
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-17 , DOI: 10.1021/jacs.0c04914 Vladislav G Lisnyak 1 , Scott A Snyder 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-06-17 , DOI: 10.1021/jacs.0c04914 Vladislav G Lisnyak 1 , Scott A Snyder 1
Affiliation
|
Among defensive alkaloids isolated from ladybugs, the heterodimeric member chilocorine C possesses an alluring monomeric unit that combines quinolizidine and indolizidine substructures. Indeed, the overall stereochemical disposition of its ring fusions is distinct from related natural products. Herein, we show that a carefully orchestrated sequence with several chemoselective transformations, including a designed cascade that accomplishes 9 distinct chemical reactions in one pot, can smoothly forge that domain and ultimately enable a 15-step, 11-pot enantiospecific synthesis of the natural product. Mechanistic studies, DFT calculations, and the delineation of several other unsuccessful approaches highlight its unique elements.
中文翻译:

以还原环化/曼尼希反应级联为燃料的 Chilocorine C 的简洁、对映特异性全合成
在从瓢虫中分离出的防御性生物碱中,异二聚体成员 chilocorine C 拥有一个诱人的单体单元,该单元结合了喹尼西啶和吲哚里西啶亚结构。事实上,其环融合体的整体立体化学分布不同于相关的天然产物。在这里,我们展示了一个精心编排的序列与几个化学选择性转化,包括一个设计的级联,在一个锅中完成 9 个不同的化学反应,可以顺利地构建该域,并最终实现天然产物的 15 步、11 锅对映特异性合成. 机理研究、DFT 计算以及其他几种不成功方法的描述突出了其独特的元素。
更新日期:2020-06-17
中文翻译:

以还原环化/曼尼希反应级联为燃料的 Chilocorine C 的简洁、对映特异性全合成
在从瓢虫中分离出的防御性生物碱中,异二聚体成员 chilocorine C 拥有一个诱人的单体单元,该单元结合了喹尼西啶和吲哚里西啶亚结构。事实上,其环融合体的整体立体化学分布不同于相关的天然产物。在这里,我们展示了一个精心编排的序列与几个化学选择性转化,包括一个设计的级联,在一个锅中完成 9 个不同的化学反应,可以顺利地构建该域,并最终实现天然产物的 15 步、11 锅对映特异性合成. 机理研究、DFT 计算以及其他几种不成功方法的描述突出了其独特的元素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号