当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CRISPR-dCas9 Mediated Cytosine Deaminase Base Editing in Bacillus subtilis.
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-06-17 , DOI: 10.1021/acssynbio.0c00151 Sili Yu 1, 2, 3 , Marcus A Price 4 , Yu Wang 1, 2, 3 , Yang Liu 2, 3 , Yanmei Guo 2, 3 , Xiaomeng Ni 2, 3 , Susan J Rosser 4 , Changhao Bi 1, 2, 3 , Meng Wang 1, 2, 3
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-06-17 , DOI: 10.1021/acssynbio.0c00151 Sili Yu 1, 2, 3 , Marcus A Price 4 , Yu Wang 1, 2, 3 , Yang Liu 2, 3 , Yanmei Guo 2, 3 , Xiaomeng Ni 2, 3 , Susan J Rosser 4 , Changhao Bi 1, 2, 3 , Meng Wang 1, 2, 3
Affiliation
|
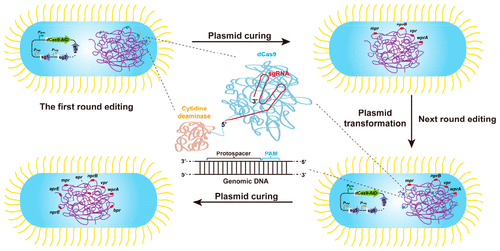
Base editing technology based on clustered regularly interspaced short palindromic repeats/associated protein 9 (CRISPR/Cas9) is a recent addition to the family of CRISPR technologies. Compared with the traditional CRISPR/Cas9 technology, it does not rely on DNA double strand break and homologous recombination, and can realize gene inactivation and point mutation more quickly and simply. Herein, we first developed a base editing method for genome editing in Bacillus subtilis utilizing CRISPR/dCas9 (a fully nuclease-deficient mutant of Cas9 from S. pyogenes) and activation-induced cytidine deaminase (AID). This method achieved three and four loci simultaneous editing with editing efficiency up to 100% and 50%, respectively. Our base editing system in B. subtilis has a 5 nt editing window, which is similar to previously reported base editing in other microorganisms. We demonstrated that the plasmid curing rate is almost 100%, which is advantageous for multiple rounds of genome engineering in B. subtilis. Finally, we applied multiplex genome editing to generate a B. subtilis 168 mutant strain with eight inactive extracellular protease genes in just two rounds of base editing and plasmid curing, suggesting that it is a powerful tool for gene manipulation in B. subtilis and industrial applications in the future.
中文翻译:

枯草芽孢杆菌中的 CRISPR-dCas9 介导的胞嘧啶脱氨酶碱基编辑。
基于成簇的规则间隔短回文重复序列/相关蛋白 9 (CRISPR/Cas9) 的碱基编辑技术是 CRISPR 技术家族的最新成员。与传统的CRISPR/Cas9技术相比,它不依赖DNA双链断裂和同源重组,可以更快速简单地实现基因失活和点突变。在此,我们首先开发了一种碱基编辑方法,利用 CRISPR/dCas9(来自化脓性链球菌的 Cas9 的完全核酸酶缺陷突变体)和活化诱导的胞苷脱氨酶 (AID)在枯草芽孢杆菌中进行基因组编辑。该方法实现了三个和四个位点同时编辑,编辑效率分别高达 100% 和 50%。我们在枯草芽孢杆菌中的碱基编辑系统有一个 5 nt 的编辑窗口,这与之前报道的其他微生物中的碱基编辑相似。我们证明了质粒治愈率几乎是 100%,这有利于枯草芽孢杆菌的多轮基因组工程。最后,我们应用多重基因组编辑在两轮碱基编辑和质粒固化中生成了具有 8 个无活性胞外蛋白酶基因的枯草芽孢杆菌168 突变菌株,表明它是枯草芽孢杆菌基因操作和工业应用的有力工具将来。
更新日期:2020-07-17
中文翻译:

枯草芽孢杆菌中的 CRISPR-dCas9 介导的胞嘧啶脱氨酶碱基编辑。
基于成簇的规则间隔短回文重复序列/相关蛋白 9 (CRISPR/Cas9) 的碱基编辑技术是 CRISPR 技术家族的最新成员。与传统的CRISPR/Cas9技术相比,它不依赖DNA双链断裂和同源重组,可以更快速简单地实现基因失活和点突变。在此,我们首先开发了一种碱基编辑方法,利用 CRISPR/dCas9(来自化脓性链球菌的 Cas9 的完全核酸酶缺陷突变体)和活化诱导的胞苷脱氨酶 (AID)在枯草芽孢杆菌中进行基因组编辑。该方法实现了三个和四个位点同时编辑,编辑效率分别高达 100% 和 50%。我们在枯草芽孢杆菌中的碱基编辑系统有一个 5 nt 的编辑窗口,这与之前报道的其他微生物中的碱基编辑相似。我们证明了质粒治愈率几乎是 100%,这有利于枯草芽孢杆菌的多轮基因组工程。最后,我们应用多重基因组编辑在两轮碱基编辑和质粒固化中生成了具有 8 个无活性胞外蛋白酶基因的枯草芽孢杆菌168 突变菌株,表明它是枯草芽孢杆菌基因操作和工业应用的有力工具将来。


























 京公网安备 11010802027423号
京公网安备 11010802027423号