当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Highly Efficient Dual‐AAV Split Adenosine Base Editor for In Vivo Gene Therapy
Small Methods ( IF 12.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/smtd.202000309 Yuxi Chen 1, 2 , Shengyao Zhi 1 , Weiliang Liu 1 , Jinkun Wen 1, 3 , Sihui Hu 1 , Tianqi Cao 1 , Hongwei Sun 1 , Yang Li 1 , Li Huang 2 , Yizhi Liu 2 , Puping Liang 1 , Junjiu Huang 1, 4
Small Methods ( IF 12.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/smtd.202000309 Yuxi Chen 1, 2 , Shengyao Zhi 1 , Weiliang Liu 1 , Jinkun Wen 1, 3 , Sihui Hu 1 , Tianqi Cao 1 , Hongwei Sun 1 , Yang Li 1 , Li Huang 2 , Yizhi Liu 2 , Puping Liang 1 , Junjiu Huang 1, 4
Affiliation
|
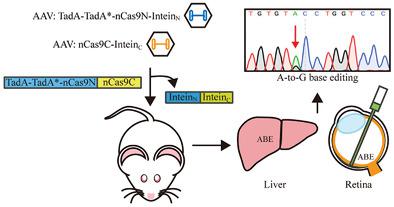
The adenosine base editor (ABE) is able to catalyze A•T to C•G conversion efficiently and precisely in vivo, representing a new method for gene therapy. Adeno associated virus (AAV) is a well‐studied vector for gene delivery in vivo. However, due to the limited loading capacity of AAV vector (≈4800 bp), it is difficult to package ABE (≈5400 bp) into a single AAV. To tackle this problem, ABE can be split into two smaller parts through intein‐mediated protein trans‐splicing. Here, 14 different split sites of nCas9 (Cas9 nickase) in combination with three different inteins (Mxe, Npu, and Rma) are screened through a GFP‐based reporter system to identify novel split‐ABEs. After infecting HEK293T and HeLa cells with dual AAVs, two split‐ABEs (split‐ABE‐Rma573 and split‐ABE‐Rma674) that can edit the target gene efficiently are identified. Furthermore, these dual‐AAV split‐ABEs can effectively disrupt the splicing acceptor of PCSK9 in mouse liver and the splicing donor of NR2E3 in mouse retina through AI‐MAST strategy. This study provides two new split‐ABEs to investigate gene function in vivo and in gene therapy, representing a new method to treat diseases by precisely repairing point mutations or inactivating genes through the AI‐MAST strategy.
中文翻译:

用于体内基因治疗的高效Dual-AAV拆分腺苷碱基编辑器的开发
腺苷碱基编辑器(ABE)能够在体内高效,精确地催化A•T向C•G的转化,代表了一种基因治疗的新方法。腺相关病毒(AAV)是在体内基因传递方面经过充分研究的载体。但是,由于AAV矢量的有限加载能力(≈4800bp),很难将ABE(≈5400bp)包装到单个AAV中。为了解决这个问题,可以通过内含子介导的蛋白质转座将ABE分为两个较小的部分。在这里,通过基于GFP的报告系统筛选了nCas9(Cas9切口酶)的14个不同的分裂位点与三种不同的内含蛋白(Mxe,Npu和Rma)结合,以鉴定新的split-ABE。用双重AAV感染HEK293T和HeLa细胞后,确定了可以有效编辑靶基因的两个split-ABE(split-ABE-Rma573和split-ABE-Rma674)。此外,通过AI-MAST策略在小鼠肝脏中PCSK9和在小鼠视网膜中NR2E3的剪接供体。这项研究提供了两个新的split-ABE,用于研究体内和基因治疗中的基因功能,代表了一种通过精确修复点突变或通过AI-MAST策略灭活基因来治疗疾病的新方法。
更新日期:2020-06-18
中文翻译:

用于体内基因治疗的高效Dual-AAV拆分腺苷碱基编辑器的开发
腺苷碱基编辑器(ABE)能够在体内高效,精确地催化A•T向C•G的转化,代表了一种基因治疗的新方法。腺相关病毒(AAV)是在体内基因传递方面经过充分研究的载体。但是,由于AAV矢量的有限加载能力(≈4800bp),很难将ABE(≈5400bp)包装到单个AAV中。为了解决这个问题,可以通过内含子介导的蛋白质转座将ABE分为两个较小的部分。在这里,通过基于GFP的报告系统筛选了nCas9(Cas9切口酶)的14个不同的分裂位点与三种不同的内含蛋白(Mxe,Npu和Rma)结合,以鉴定新的split-ABE。用双重AAV感染HEK293T和HeLa细胞后,确定了可以有效编辑靶基因的两个split-ABE(split-ABE-Rma573和split-ABE-Rma674)。此外,通过AI-MAST策略在小鼠肝脏中PCSK9和在小鼠视网膜中NR2E3的剪接供体。这项研究提供了两个新的split-ABE,用于研究体内和基因治疗中的基因功能,代表了一种通过精确修复点突变或通过AI-MAST策略灭活基因来治疗疾病的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号