当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rheumatoid arthritis synovial microenvironment induces metabolic and functional adaptations in dendritic cells.
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-06-17 , DOI: 10.1111/cei.13479 M Canavan 1, 2 , V Marzaioli 1, 2 , T McGarry 1 , V Bhargava 3 , S Nagpal 3 , D J Veale 2 , U Fearon 1, 2
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-06-17 , DOI: 10.1111/cei.13479 M Canavan 1, 2 , V Marzaioli 1, 2 , T McGarry 1 , V Bhargava 3 , S Nagpal 3 , D J Veale 2 , U Fearon 1, 2
Affiliation
|
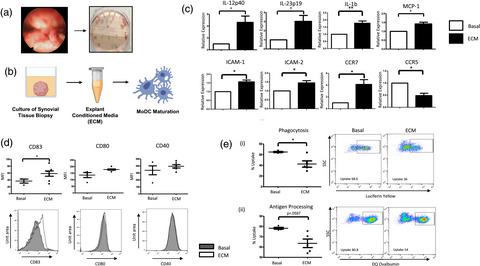
Rheumatoid arthritis (RA) is a chronic autoimmune disease which causes degradation of cartilage and bone. It is well appreciated that the pathogenic hallmark of RA is the mass influx of inflammatory cells into the joint. However, the role that dendritic cells (DC) may play in this inflammatory milieu is still relatively unexplored. Moreover, the contribution this unique synovial microenvironment has on DC maturation is still unknown. Using monocyte‐derived DC (MoDC), we established an in‐vitro model to recapitulate the synovial microenvironment to explore DC maturation. MoDC treated with conditioned media from ex‐vivo synovial tissue biopsy cultures [explant‐conditioned media (ECM)] have increased expression of proinflammatory cytokines, chemokines and adhesion molecules. ECM DC have increased expression of CD83 and CC‐chemokine receptor (CCR)7 and decreased expression of CCR5 and phagocytic capacity, suggestive of heightened DC maturation. ECM‐induced maturation is concomitant with altered cellular bioenergetics, whereby increased expression of glycolytic genes and increased glucose uptake are observed in ECM DC. Collectively, this results in a metabolic shift in DC metabolism in favour of glycolysis. These adaptations are in‐part mediated via signal transducer and activator of transcription‐3 (STAT‐3), as demonstrated by decreased expression of proinflammatory cytokines and glycolytic genes in ECM DC in response to STAT‐3 inhibition. Finally, to translate these data to a more in‐vivo clinically relevant setting, RNA‐seq was performed on RA synovial fluid and peripheral blood. We identified enhanced expression of a number of glycolytic genes in synovial CD1c+ DC compared to CD1c+ DC in circulation. Collectively, our data suggest that the synovial microenvironment in RA contributes to DC maturation and metabolic reprogramming.
中文翻译:

类风湿性关节炎滑膜微环境诱导树突细胞的代谢和功能适应。
类风湿性关节炎 (RA) 是一种慢性自身免疫性疾病,会导致软骨和骨骼退化。众所周知,RA 的致病标志是炎症细胞大量流入关节。然而,树突状细胞 (DC) 在这种炎症环境中可能发挥的作用仍然相对未探索。此外,这种独特的滑膜微环境对 DC 成熟的贡献仍然未知。使用单核细胞衍生的 DC(MoDC),我们建立了一个体外模型来重现滑膜微环境以探索 DC 的成熟。用离体条件培养基处理的 MoDC滑膜组织活检培养物 [外植体条件培养基 (ECM)] 增加了促炎细胞因子、趋化因子和粘附分子的表达。ECM DC 增加了 CD83 和 CC-趋化因子受体 (CCR)7 的表达,降低了 CCR5 的表达和吞噬能力,表明 DC 成熟度提高。ECM 诱导的成熟伴随着细胞生物能量学的改变,因此在 ECM DC 中观察到糖酵解基因表达增加和葡萄糖摄取增加。总的来说,这导致 DC 代谢发生有利于糖酵解的代谢转变。这些适应部分是通过信号转导和转录激活因子 3 (STAT-3) 介导的,如 STAT-3 抑制导致 ECM DC 中促炎细胞因子和糖酵解基因表达降低所证明的那样。最后,在体内临床相关设置中,对 RA 滑液和外周血进行 RNA-seq。我们鉴定增强的一个数糖酵解基因的表达滑膜CD1C + DC相比CD1C + DC在流通。总的来说,我们的数据表明 RA 的滑膜微环境有助于 DC 成熟和代谢重编程。
更新日期:2020-06-17
中文翻译:

类风湿性关节炎滑膜微环境诱导树突细胞的代谢和功能适应。
类风湿性关节炎 (RA) 是一种慢性自身免疫性疾病,会导致软骨和骨骼退化。众所周知,RA 的致病标志是炎症细胞大量流入关节。然而,树突状细胞 (DC) 在这种炎症环境中可能发挥的作用仍然相对未探索。此外,这种独特的滑膜微环境对 DC 成熟的贡献仍然未知。使用单核细胞衍生的 DC(MoDC),我们建立了一个体外模型来重现滑膜微环境以探索 DC 的成熟。用离体条件培养基处理的 MoDC滑膜组织活检培养物 [外植体条件培养基 (ECM)] 增加了促炎细胞因子、趋化因子和粘附分子的表达。ECM DC 增加了 CD83 和 CC-趋化因子受体 (CCR)7 的表达,降低了 CCR5 的表达和吞噬能力,表明 DC 成熟度提高。ECM 诱导的成熟伴随着细胞生物能量学的改变,因此在 ECM DC 中观察到糖酵解基因表达增加和葡萄糖摄取增加。总的来说,这导致 DC 代谢发生有利于糖酵解的代谢转变。这些适应部分是通过信号转导和转录激活因子 3 (STAT-3) 介导的,如 STAT-3 抑制导致 ECM DC 中促炎细胞因子和糖酵解基因表达降低所证明的那样。最后,在体内临床相关设置中,对 RA 滑液和外周血进行 RNA-seq。我们鉴定增强的一个数糖酵解基因的表达滑膜CD1C + DC相比CD1C + DC在流通。总的来说,我们的数据表明 RA 的滑膜微环境有助于 DC 成熟和代谢重编程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号