当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sulphonamidic Groups as Electron-Withdrawing Units in Ureido-Based Anion Receptors: Enhanced Anion Complexation versus Deprotonation.
ChemPlusChem ( IF 3.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/cplu.202000326 Karolína Salvadori 1, 2, 3 , Ludmila Šimková 2 , Ivana Císařová 4 , Jan Sýkora 1 , Jiří Ludvík 2 , Petra Cuřínová 1
ChemPlusChem ( IF 3.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/cplu.202000326 Karolína Salvadori 1, 2, 3 , Ludmila Šimková 2 , Ivana Císařová 4 , Jan Sýkora 1 , Jiří Ludvík 2 , Petra Cuřínová 1
Affiliation
|
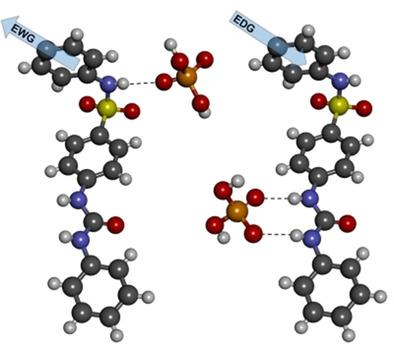
A sulphonamidic moiety was utilized as an electron‐withdrawing group for enhancement of anion complexation features of urea‐based receptors. A series of receptors varying in acidity of sulphonamidic and urea NH groups was synthesized and thoroughly tested. The individual complexation properties reflect deprotonation/complexation equilibrium in a given molecule as a function of the substitution. The receptors containing electron‐donating groups in conjugation to the sulphonamidic moiety showed higher association constants towards H2PO4− and carboxylate anions, while those containing electron‐withdrawing groups inclined to deprotonation of sulphonamidic NH . The deprotonation issue can be avoided by alkylation at the early step of receptor synthesis or it can be utilized for insertion of suitable groups that enable its anchoring on various substrates to form more elaborated receptor structures.
中文翻译:

脲基基团作为基于Ureido的阴离子受体中的电子吸收单元:增强的阴离子络合与去质子化。
磺酰胺基团被用作吸电子基团,以增强基于尿素的受体的阴离子络合特征。合成并彻底测试了一系列磺酰胺基和尿素NH基团的酸度变化的受体。各个络合性质反映了给定分子中作为取代的函数的去质子/络合平衡。含有缀合sulphonamidic部分给电子基团的受体显示出朝向h时缔合常数2 PO 4 -和羧酸根阴离子,而那些含有吸电子基团倾向于sulphonamidic N中的去质子化ħ。可以通过在受体合成的早期阶段进行烷基化来避免去质子化问题,也可以将其用于插入合适的基团,从而使其锚定在各种底物上,从而形成更精细的受体结构。
更新日期:2020-07-03
中文翻译:

脲基基团作为基于Ureido的阴离子受体中的电子吸收单元:增强的阴离子络合与去质子化。
磺酰胺基团被用作吸电子基团,以增强基于尿素的受体的阴离子络合特征。合成并彻底测试了一系列磺酰胺基和尿素NH基团的酸度变化的受体。各个络合性质反映了给定分子中作为取代的函数的去质子/络合平衡。含有缀合sulphonamidic部分给电子基团的受体显示出朝向h时缔合常数2 PO 4 -和羧酸根阴离子,而那些含有吸电子基团倾向于sulphonamidic N中的去质子化ħ。可以通过在受体合成的早期阶段进行烷基化来避免去质子化问题,也可以将其用于插入合适的基团,从而使其锚定在各种底物上,从而形成更精细的受体结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号