当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fit-For-Purpose Protein Biomarker Assay Validation Strategies Using Hybrid Immunocapture-Liquid Chromatography-Tandem-Mass Spectrometry Platform: Quantitative Analysis of Total Soluble Cluster of differentiation 73
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.aca.2020.06.023 Yue Zhao 1 , Huidong Gu 1 , Jennifer Postelnek 1 , Marissa DeMichele 1 , Long Yuan 1 , Yan J Zhang 1 , Jianing Zeng 1
Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.aca.2020.06.023 Yue Zhao 1 , Huidong Gu 1 , Jennifer Postelnek 1 , Marissa DeMichele 1 , Long Yuan 1 , Yan J Zhang 1 , Jianing Zeng 1
Affiliation
|
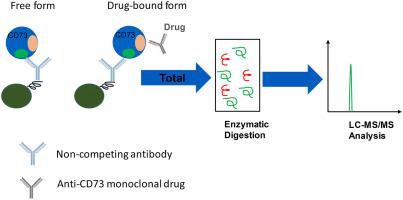
In recent years, biomarkers have played more extensive roles as indicators of disease progression, safety, and drug efficacy. Targeted quantitative analysis of biomarkers including drug targets have become increasingly important to drive critical decision-making in various drug development stages, as well as to improve the success rates of clinical trials. There are many analytical challenges when developing and validating the bioanalytical methods associated with the measurement of an endogenous protein biomarker, especially when using LC-MS based analysis. Moreover, the current regulatory guidelines for assay development and validation using LC-MS platform mainly focuse on regulated bioanalysis for therapeutic drugs. In this manuscript, we use total soluble CD73 (sCD73) as an example to present a "fit-for-purpose" assay using a hybrid immunocapture-LC-MS/MS assay platform. A non-competing antibody (to the therapeutic drug) was used to isolate and enrich the total sCD73 from biological matrix. The enriched sample was digested after immunocapture and a surrogate peptide was monitored for quantification. The assay showed good accuracy, precision, specificity and sensitivity with the LLOQ of 1.00 ng/mL, and was applied in a clinical study to measure the total sCD73 as a potential pharmacodynamic (PD) marker. Some recommendations and considerations for "fit-for-purpose" validation of this assay, and hybrid LC-MS assays in general, for the quantitative analysis of an endogenous protein biomarkers is also discussed.
中文翻译:

使用混合免疫捕获-液相色谱-串联质谱平台的适合用途的蛋白质生物标志物检测验证策略:总可溶性分化簇的定量分析 73
近年来,生物标志物作为疾病进展、安全性和药物疗效的指标发挥了更广泛的作用。对包括药物靶点在内的生物标志物进行有针对性的定量分析对于推动各个药物开发阶段的关键决策以及提高临床试验的成功率变得越来越重要。在开发和验证与内源性蛋白质生物标志物测量相关的生物分析方法时,存在许多分析挑战,尤其是在使用基于 LC-MS 的分析时。此外,目前使用 LC-MS 平台进行检测开发和验证的监管指南主要侧重于治疗药物的监管生物分析。在这份手稿中,我们以总可溶性 CD73 (sCD73) 为例来展示“适合目的” 使用混合免疫捕获-LC-MS/MS 分析平台进行分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。具有 1.00 ng/mL LLOQ 的特异性和敏感性,并被应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。具有 1.00 ng/mL LLOQ 的特异性和敏感性,并被应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。
更新日期:2020-08-01
中文翻译:

使用混合免疫捕获-液相色谱-串联质谱平台的适合用途的蛋白质生物标志物检测验证策略:总可溶性分化簇的定量分析 73
近年来,生物标志物作为疾病进展、安全性和药物疗效的指标发挥了更广泛的作用。对包括药物靶点在内的生物标志物进行有针对性的定量分析对于推动各个药物开发阶段的关键决策以及提高临床试验的成功率变得越来越重要。在开发和验证与内源性蛋白质生物标志物测量相关的生物分析方法时,存在许多分析挑战,尤其是在使用基于 LC-MS 的分析时。此外,目前使用 LC-MS 平台进行检测开发和验证的监管指南主要侧重于治疗药物的监管生物分析。在这份手稿中,我们以总可溶性 CD73 (sCD73) 为例来展示“适合目的” 使用混合免疫捕获-LC-MS/MS 分析平台进行分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。使用非竞争性抗体(针对治疗药物)从生物基质中分离和富集总 sCD73。在免疫捕获后消化富集的样品并监测替代肽的定量。该测定显示出良好的准确度、精密度、特异性和灵敏度,LLOQ 为 1.00 ng/mL,并应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。具有 1.00 ng/mL LLOQ 的特异性和敏感性,并被应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。具有 1.00 ng/mL LLOQ 的特异性和敏感性,并被应用于临床研究以测量总 sCD73 作为潜在的药效学 (PD) 标志物。还讨论了对该测定法的“适合用途”验证的一些建议和注意事项,以及一般的混合 LC-MS 测定法,用于内源性蛋白质生物标志物的定量分析。

























 京公网安备 11010802027423号
京公网安备 11010802027423号