当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New inhibitors for the BPTF bromodomain enabled by structural biology and biophysical assay development.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0ob00506a Peter D Ycas 1 , Huda Zahid 1 , Alice Chan 2 , Noelle M Olson 1 , Jorden A Johnson 1 , Siva K Talluri 1 , Ernst Schonbrunn 2 , William C K Pomerantz 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0ob00506a Peter D Ycas 1 , Huda Zahid 1 , Alice Chan 2 , Noelle M Olson 1 , Jorden A Johnson 1 , Siva K Talluri 1 , Ernst Schonbrunn 2 , William C K Pomerantz 1
Affiliation
|
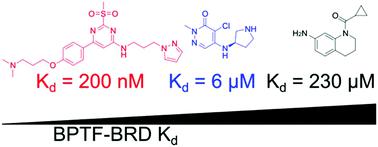
Bromodomain-containing proteins regulate transcription through protein–protein interactions with chromatin and serve as scaffolding proteins for recruiting essential members of the transcriptional machinery. One such protein is the bromodomain and PHD-containing transcription factor (BPTF), the largest member of the nucleosome remodeling complex, NURF. Despite an emerging role for BPTF in regulating a diverse set of cancers, small molecule development for inhibiting the BPTF bromodomain has been lacking. Here we cross-validate three complementary biophysical assays to further the discovery of BPTF bromodomain inhibitors for chemical probe development: two direct binding assays (protein-observed 19F (PrOF) NMR and surface plasmon resonance (SPR)) and a competitive inhibition assay (AlphaScreen). We first compare the assays using three small molecules and acetylated histone peptides with reported affinity for the BPTF bromodomain. Using SPR with both unlabeled and fluorinated BPTF, we further determine that there is a minimal effect of 19F incorporation on ligand binding for future PrOF NMR experiments. To guide medicinal chemistry efforts towards chemical probe development, we subsequently evaluate two new BPTF inhibitor scaffolds with our suite of biophysical assays and rank-order compound affinities which could not otherwise be determined by PrOF NMR. Finally, we cocrystallize a subset of small molecule inhibitors and present the first published small molecule-protein structures with the BPTF bromodomain. We envision the biophysical assays described here and the structural insights from the crystallography will guide researchers towards developing selective and potent BPTF bromodomain inhibitors.
中文翻译:

通过结构生物学和生物物理检测开发实现 BPTF 溴结构域的新抑制剂。
含溴结构域的蛋白质通过与染色质的蛋白质-蛋白质相互作用来调节转录,并充当招募转录机制的重要成员的支架蛋白。其中一种蛋白质是含溴结构域和 PHD 的转录因子 (BPTF),它是核小体重塑复合物 NURF 的最大成员。尽管 BPTF 在调节多种癌症方面发挥着新兴作用,但抑制 BPTF 溴结构域的小分子开发一直缺乏。在这里,我们交叉验证了三种互补的生物物理测定,以进一步发现用于化学探针开发的 BPTF 溴结构域抑制剂:两种直接结合测定(蛋白质观察19 F (PrOF) NMR 和表面等离子共振 (SPR))和竞争性抑制测定(阿尔法屏幕)。我们首先比较使用三种小分子和乙酰化组蛋白肽的测定,并报告对 BPTF 溴结构域的亲和力。使用具有未标记和氟化 BPTF 的 SPR,我们进一步确定19 F 掺入对未来 PrOF NMR 实验的配体结合的影响最小。为了指导化学探针开发的药物化学工作,我们随后使用我们的一套生物物理测定和排序化合物亲和力评估了两种新的 BPTF 抑制剂支架,否则无法通过 PrOF NMR 确定。最后,我们共结晶了小分子抑制剂的子集,并提出了第一个发表的小分子蛋白质结构与 BPTF 溴结构域。我们设想这里描述的生物物理测定和晶体学的结构见解将指导研究人员开发选择性和有效的 BPTF 溴结构域抑制剂。
更新日期:2020-07-15
中文翻译:

通过结构生物学和生物物理检测开发实现 BPTF 溴结构域的新抑制剂。
含溴结构域的蛋白质通过与染色质的蛋白质-蛋白质相互作用来调节转录,并充当招募转录机制的重要成员的支架蛋白。其中一种蛋白质是含溴结构域和 PHD 的转录因子 (BPTF),它是核小体重塑复合物 NURF 的最大成员。尽管 BPTF 在调节多种癌症方面发挥着新兴作用,但抑制 BPTF 溴结构域的小分子开发一直缺乏。在这里,我们交叉验证了三种互补的生物物理测定,以进一步发现用于化学探针开发的 BPTF 溴结构域抑制剂:两种直接结合测定(蛋白质观察19 F (PrOF) NMR 和表面等离子共振 (SPR))和竞争性抑制测定(阿尔法屏幕)。我们首先比较使用三种小分子和乙酰化组蛋白肽的测定,并报告对 BPTF 溴结构域的亲和力。使用具有未标记和氟化 BPTF 的 SPR,我们进一步确定19 F 掺入对未来 PrOF NMR 实验的配体结合的影响最小。为了指导化学探针开发的药物化学工作,我们随后使用我们的一套生物物理测定和排序化合物亲和力评估了两种新的 BPTF 抑制剂支架,否则无法通过 PrOF NMR 确定。最后,我们共结晶了小分子抑制剂的子集,并提出了第一个发表的小分子蛋白质结构与 BPTF 溴结构域。我们设想这里描述的生物物理测定和晶体学的结构见解将指导研究人员开发选择性和有效的 BPTF 溴结构域抑制剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号