当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic reduction of CO2 into fuels and fine chemicals
Green Chemistry ( IF 9.8 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0gc01092h Arindam Modak 1, 2, 3, 4, 5 , Piyali Bhanja 5, 6, 7, 8 , Saikat Dutta 8, 9, 10, 11, 12 , Biswajit Chowdhury 8, 13, 14, 15 , Asim Bhaumik 5, 6, 7, 8
Green Chemistry ( IF 9.8 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0gc01092h Arindam Modak 1, 2, 3, 4, 5 , Piyali Bhanja 5, 6, 7, 8 , Saikat Dutta 8, 9, 10, 11, 12 , Biswajit Chowdhury 8, 13, 14, 15 , Asim Bhaumik 5, 6, 7, 8
Affiliation
|
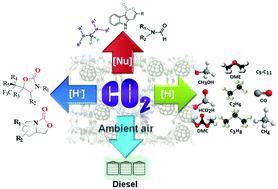
With the progressive increase in atmospheric CO2 over the years and owing to its potential environmental threat, researchers have focused their attention on the fruitful utilization of CO2 into value-added chemicals and feedstocks. Although CO2 conversion reactions are intensively studied through several electrochemical, photochemical and photo-electrochemical pathways, chemical reduction of CO2 is more challenging to achieve due to the involvement of breaking of high energy C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.
O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.
中文翻译:

将二氧化碳催化还原为燃料和精细化学品
多年来,随着大气中的CO 2逐渐增加以及由于其潜在的环境威胁,研究人员将注意力集中在将CO 2有效地用于增值化学品和原料中。尽管已通过几种电化学,光化学和光电化学途径对CO 2转化反应进行了深入研究,但由于涉及高能C O键的断裂而没有任何施加电势的参与以及CO的完成,因此实现CO 2的化学还原更具挑战性![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 。绿色化学观点。化学还原CO 2方面的重大进展这些年来,在还原剂的存在下,均相催化剂和非均相催化剂已经获得了成功。但是,在将该技术用于大规模工业过程之前,有许多陷阱需要克服。我们在这里展示了将CO 2还原为基本燃料[CO,CH 3 OH,CH 3 CH 2 OH,HCO 2 H,CH 4,二甲醚(DME),碳酸二甲酯(DMC)和低级烃]的最新进展以及有价值的化学物质通过亲核加成反应。我们还强调了CO 2的直接转化解决环境中碳排放问题的可能路线图,这些气体来自极稀释的来源(例如环境空气)。整个讨论分为两个部分,在第一部分中,我们总结了涉及亲核加成CO 2的几种均相催化过程,导致形成C–C和C–H键,从而导致了2-恶唑烷酮,缩醛胺,末端的合成。羧化产物和吲哚内酯衍生物,可能对制药业有利。还使用沮丧的路易斯对(FLP)作为催化剂列出了其他还原产物,例如甲烷,甲醇和甲醇盐。第二部分广泛强调了用H 2还原CO 2的非均相催化剂。然而,通过司法考虑反应的热力学和动力学,仍需要大量努力来在试验工厂规模上开发出活性,选择性和稳定的催化剂。CO 2的还原可以在无机载体(CeO 2,Al 2 O 3,TiO 2等)和纳米结构的多孔骨架(沸石,多孔聚合物,中孔二氧化硅)上固定的金属纳米颗粒上进行。因此,有必要对涉及不同活性部位的整个过程的反应机理进行深入研究。首先,本次综述汇集了CO 2的主要进步还原过程以及对实现CO 2分子活化的实用性和挑战的关注。
。绿色化学观点。化学还原CO 2方面的重大进展这些年来,在还原剂的存在下,均相催化剂和非均相催化剂已经获得了成功。但是,在将该技术用于大规模工业过程之前,有许多陷阱需要克服。我们在这里展示了将CO 2还原为基本燃料[CO,CH 3 OH,CH 3 CH 2 OH,HCO 2 H,CH 4,二甲醚(DME),碳酸二甲酯(DMC)和低级烃]的最新进展以及有价值的化学物质通过亲核加成反应。我们还强调了CO 2的直接转化解决环境中碳排放问题的可能路线图,这些气体来自极稀释的来源(例如环境空气)。整个讨论分为两个部分,在第一部分中,我们总结了涉及亲核加成CO 2的几种均相催化过程,导致形成C–C和C–H键,从而导致了2-恶唑烷酮,缩醛胺,末端的合成。羧化产物和吲哚内酯衍生物,可能对制药业有利。还使用沮丧的路易斯对(FLP)作为催化剂列出了其他还原产物,例如甲烷,甲醇和甲醇盐。第二部分广泛强调了用H 2还原CO 2的非均相催化剂。然而,通过司法考虑反应的热力学和动力学,仍需要大量努力来在试验工厂规模上开发出活性,选择性和稳定的催化剂。CO 2的还原可以在无机载体(CeO 2,Al 2 O 3,TiO 2等)和纳米结构的多孔骨架(沸石,多孔聚合物,中孔二氧化硅)上固定的金属纳米颗粒上进行。因此,有必要对涉及不同活性部位的整个过程的反应机理进行深入研究。首先,本次综述汇集了CO 2的主要进步还原过程以及对实现CO 2分子活化的实用性和挑战的关注。
更新日期:2020-07-06
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.
O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.
中文翻译:

将二氧化碳催化还原为燃料和精细化学品
多年来,随着大气中的CO 2逐渐增加以及由于其潜在的环境威胁,研究人员将注意力集中在将CO 2有效地用于增值化学品和原料中。尽管已通过几种电化学,光化学和光电化学途径对CO 2转化反应进行了深入研究,但由于涉及高能C O键的断裂而没有任何施加电势的参与以及CO的完成,因此实现CO 2的化学还原更具挑战性
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 。绿色化学观点。化学还原CO 2方面的重大进展这些年来,在还原剂的存在下,均相催化剂和非均相催化剂已经获得了成功。但是,在将该技术用于大规模工业过程之前,有许多陷阱需要克服。我们在这里展示了将CO 2还原为基本燃料[CO,CH 3 OH,CH 3 CH 2 OH,HCO 2 H,CH 4,二甲醚(DME),碳酸二甲酯(DMC)和低级烃]的最新进展以及有价值的化学物质通过亲核加成反应。我们还强调了CO 2的直接转化解决环境中碳排放问题的可能路线图,这些气体来自极稀释的来源(例如环境空气)。整个讨论分为两个部分,在第一部分中,我们总结了涉及亲核加成CO 2的几种均相催化过程,导致形成C–C和C–H键,从而导致了2-恶唑烷酮,缩醛胺,末端的合成。羧化产物和吲哚内酯衍生物,可能对制药业有利。还使用沮丧的路易斯对(FLP)作为催化剂列出了其他还原产物,例如甲烷,甲醇和甲醇盐。第二部分广泛强调了用H 2还原CO 2的非均相催化剂。然而,通过司法考虑反应的热力学和动力学,仍需要大量努力来在试验工厂规模上开发出活性,选择性和稳定的催化剂。CO 2的还原可以在无机载体(CeO 2,Al 2 O 3,TiO 2等)和纳米结构的多孔骨架(沸石,多孔聚合物,中孔二氧化硅)上固定的金属纳米颗粒上进行。因此,有必要对涉及不同活性部位的整个过程的反应机理进行深入研究。首先,本次综述汇集了CO 2的主要进步还原过程以及对实现CO 2分子活化的实用性和挑战的关注。
。绿色化学观点。化学还原CO 2方面的重大进展这些年来,在还原剂的存在下,均相催化剂和非均相催化剂已经获得了成功。但是,在将该技术用于大规模工业过程之前,有许多陷阱需要克服。我们在这里展示了将CO 2还原为基本燃料[CO,CH 3 OH,CH 3 CH 2 OH,HCO 2 H,CH 4,二甲醚(DME),碳酸二甲酯(DMC)和低级烃]的最新进展以及有价值的化学物质通过亲核加成反应。我们还强调了CO 2的直接转化解决环境中碳排放问题的可能路线图,这些气体来自极稀释的来源(例如环境空气)。整个讨论分为两个部分,在第一部分中,我们总结了涉及亲核加成CO 2的几种均相催化过程,导致形成C–C和C–H键,从而导致了2-恶唑烷酮,缩醛胺,末端的合成。羧化产物和吲哚内酯衍生物,可能对制药业有利。还使用沮丧的路易斯对(FLP)作为催化剂列出了其他还原产物,例如甲烷,甲醇和甲醇盐。第二部分广泛强调了用H 2还原CO 2的非均相催化剂。然而,通过司法考虑反应的热力学和动力学,仍需要大量努力来在试验工厂规模上开发出活性,选择性和稳定的催化剂。CO 2的还原可以在无机载体(CeO 2,Al 2 O 3,TiO 2等)和纳米结构的多孔骨架(沸石,多孔聚合物,中孔二氧化硅)上固定的金属纳米颗粒上进行。因此,有必要对涉及不同活性部位的整个过程的反应机理进行深入研究。首先,本次综述汇集了CO 2的主要进步还原过程以及对实现CO 2分子活化的实用性和挑战的关注。


























 京公网安备 11010802027423号
京公网安备 11010802027423号