当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
mTORC1-dependent TFEB nucleus translocation and pro-survival autophagy induced by zeolitic imidazolate framework-8.
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0bm00773k He Ding 1 , Yang Song 2 , Xiaowan Huang 3 , Liansheng Wang 3 , Shanzi Luo 3 , Hao Zhang 2 , Hao Pan 3 , Wenwei Jiang 4 , Jing Qian 5 , Guangyu Yao 4 , Longping Wen 6 , Yunjiao Zhang 6
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0bm00773k He Ding 1 , Yang Song 2 , Xiaowan Huang 3 , Liansheng Wang 3 , Shanzi Luo 3 , Hao Zhang 2 , Hao Pan 3 , Wenwei Jiang 4 , Jing Qian 5 , Guangyu Yao 4 , Longping Wen 6 , Yunjiao Zhang 6
Affiliation
|
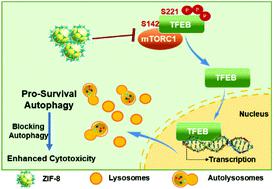
A great variety of nanoparticles are known to induce autophagy, leading to either pro-death or pro-survival. Zeolitic imidazolate framework-8 (ZIF-8), a type of porous metal–organic framework (MOF) material and a promising drug delivery vector, has reportedly shown excellent efficacy for cancer therapy. However, less attention has been paid to the potential biological effect of ZIF-8 per se, and if so, how the effect impacts cell fate and therapy outcomes. Herein, we showed that ZIF-8 induced autophagy in HeLa cells, characterized by increased autophagosome formation without disruption of autophagic flux, in a dose- and time-dependent fashion. ZIF-8 also caused dephosphorylation of the transcription factor EB (TFEB) at serine-142 and serine-211, leading to the nucleus translocation of TFEB, an event that promoted lysosome biogenesis and is necessary for autophagy induction. We further pinpointed the inhibition of mTORC1 as the critical event upstream of ZIF-8-elicited TFEB dephosphorylation and the subsequent nucleus translocation. Furthermore, autophagy induced by ZIF-8 promoted cell survival, as inhibiting autophagy by either 3-methyladenine (3-MA) or ATG5 knockdown significantly enhanced ZIF-8-elicited HeLa cell death. Most importantly, doxorubicin-encapsulated ZIF-8 (DOX@ZIF-8) also elicited strong pro-survival autophagy, and the co-delivery of an autophagic inhibitor (3-MA) dramatically enhanced the cytotoxicity of DOX@ZIF-8 in HeLa cells. Our results revealed the unique ability of ZIF-8, both in a free and drug-loaded form, to induce pro-survival autophagy in certain cancer cells, a finding with important implications for potential clinical studies that utilize ZIF-8 as a drug carrier.
中文翻译:

沸石咪唑酯框架 8 诱导的 mTORC1 依赖性 TFEB 核易位和促存活自噬。
已知多种纳米颗粒可诱导自噬,从而导致促死亡或促生存。据报道,沸石咪唑酯骨架 8 (ZIF-8) 是一种多孔金属有机骨架 (MOF) 材料和有前途的药物递送载体,据报道在癌症治疗中显示出优异的疗效。然而,对 ZIF-8本身的潜在生物学效应的关注较少,如果是这样,效果如何影响细胞命运和治疗结果。在这里,我们发现 ZIF-8 在 HeLa 细胞中诱导自噬,其特征是自噬体形成增加而不破坏自噬通量,以剂量和时间依赖性方式。ZIF-8 还引起丝氨酸 142 和丝氨酸 211 处转录因子 EB (TFEB) 的去磷酸化,导致 TFEB 的细胞核易位,这是促进溶酶体生物发生的事件,是自噬诱导所必需的。我们进一步指出 mTORC1 的抑制是 ZIF-8 引发的 TFEB 去磷酸化和随后的细胞核易位上游的关键事件。此外,ZIF-8 诱导的自噬促进细胞存活,因为 3-甲基腺嘌呤 (3-MA) 或ATG5抑制自噬敲低显着增强了 ZIF-8 引发的 HeLa 细胞死亡。最重要的是,多柔比星封装的 ZIF-8 (DOX@ZIF-8) 还引发了强烈的促生存自噬,并且自噬抑制剂 (3-MA) 的共同递送显着增强了 DOX@ZIF-8 在 HeLa 中的细胞毒性细胞。我们的研究结果揭示了 ZIF-8(无论是游离形式还是载药形式)在某些癌细胞中诱导促生存自噬的独特能力,这一发现对利用 ZIF-8 作为药物载体的潜在临床研究具有重要意义.
更新日期:2020-07-28
中文翻译:

沸石咪唑酯框架 8 诱导的 mTORC1 依赖性 TFEB 核易位和促存活自噬。
已知多种纳米颗粒可诱导自噬,从而导致促死亡或促生存。据报道,沸石咪唑酯骨架 8 (ZIF-8) 是一种多孔金属有机骨架 (MOF) 材料和有前途的药物递送载体,据报道在癌症治疗中显示出优异的疗效。然而,对 ZIF-8本身的潜在生物学效应的关注较少,如果是这样,效果如何影响细胞命运和治疗结果。在这里,我们发现 ZIF-8 在 HeLa 细胞中诱导自噬,其特征是自噬体形成增加而不破坏自噬通量,以剂量和时间依赖性方式。ZIF-8 还引起丝氨酸 142 和丝氨酸 211 处转录因子 EB (TFEB) 的去磷酸化,导致 TFEB 的细胞核易位,这是促进溶酶体生物发生的事件,是自噬诱导所必需的。我们进一步指出 mTORC1 的抑制是 ZIF-8 引发的 TFEB 去磷酸化和随后的细胞核易位上游的关键事件。此外,ZIF-8 诱导的自噬促进细胞存活,因为 3-甲基腺嘌呤 (3-MA) 或ATG5抑制自噬敲低显着增强了 ZIF-8 引发的 HeLa 细胞死亡。最重要的是,多柔比星封装的 ZIF-8 (DOX@ZIF-8) 还引发了强烈的促生存自噬,并且自噬抑制剂 (3-MA) 的共同递送显着增强了 DOX@ZIF-8 在 HeLa 中的细胞毒性细胞。我们的研究结果揭示了 ZIF-8(无论是游离形式还是载药形式)在某些癌细胞中诱导促生存自噬的独特能力,这一发现对利用 ZIF-8 作为药物载体的潜在临床研究具有重要意义.



























 京公网安备 11010802027423号
京公网安备 11010802027423号