当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Counterion Condensation, the Gibbs Equation, and Surfactant Binding: An Integrated Description of the Behavior of Polyelectrolytes and Their Mixtures with Surfactants at the Air-Water Interface.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c02988 Jeffrey Penfold 1 , Robert K Thomas 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c02988 Jeffrey Penfold 1 , Robert K Thomas 2
Affiliation
|
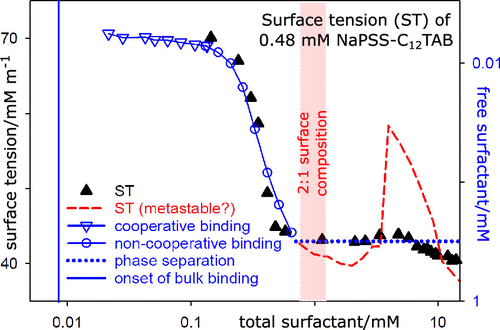
By applying the Gibbs equation to the bulk binding isotherms and surface composition of the air–water (A–W) interface in polyelectrolyte–surfactant (PE–S) systems, we show that their surface behavior can be explained semiquantitatively in terms of four concentration regions, which we label as A, B, C, and D. In the lowest-concentration range A, there are no bound PE–S complexes in the bulk but there may be adsorption of PE–S complexes at the surface. When significant adsorption occurs in this region, the surface tension (ST) drops with increasing concentration like a simple surfactant solution. Region B extends from the onset of bulk PE–S binding to the end of cooperative binding, in which the slow variation of surfactant activity with cooperative binding means that the ST changes relatively little, although adsorption may be significant. This leads to an approximate plateau, which may be at high or low ST. Region C starts where the binding in the bulk complex loses its cooperativity leading to a rapid change of surfactant activity with the total concentration. This, combined with significant adsorption, often leads to a sharp drop in ST. Region D is where precipitation and redissolution of the bulk PE–S complex occur. ST peaks may arise in region D because of loss of the solution complex that matches the value of the preferred surface stoichiometry, which seems to have a well-defined value for each system. The analysis is applied to the experimental systems, sodium polystyrene sulfonate–alkyltrimethylammonium bromides and poly(diallyldimethyl chloride)–sodium alkyl sulfates, with and without the added electrolyte, and includes data from bulk binding isotherms, phase diagrams, aggregation behavior, and direct measurements of the surface excess and stoichiometry of the surface. The successful fits of the Gibbs equation to the data confirm that the surfaces in these systems are largely equilibrated.
中文翻译:

抗衡离子冷凝,吉布斯方程和表面活性剂结合:聚电解质及其与表面活性剂在空气-水界面处的混合物的综合描述。
通过将Gibbs方程应用于聚电解质-表面活性剂(PE-S)系统中的空气-水(A-W)界面的整体结合等温线和表面组成,我们证明了它们的表面行为可以用四种浓度半定量地解释。区域,我们将其标记为A,B,C和D。在最低浓度范围A中,本体中没有键合的PE–S配合物,但表面可能吸附有PE–S配合物。当在该区域中发生大量吸附时,表面张力(ST)会像简单的表面活性剂溶液一样随着浓度的增加而下降。B区从大量PE-S结合开始到合作结合结束,其中表面活性剂活性随合作结合的缓慢变化意味着ST的变化相对较小,尽管吸附可能很明显。这导致近似的平稳期,可能处于高或低的ST。C区开始于本体复合物中的结合失去合作性,导致表面活性剂活性随总浓度快速变化。结合大量吸附,通常会导致ST急剧下降。D区是大量PE-S复合物发生沉淀和再溶解的地方。由于溶液配合物的损失与优选表面化学计量的值相匹配,似乎在每个系统中都有明确定义的值,因此在D区可能会出现ST峰。该分析适用于实验系统,聚苯乙烯磺酸钠–烷基三甲基溴化铵和聚(二烯丙基二甲基氯)–烷基硫酸钠,有或没有添加电解质,并包括本体结合等温线,相图,聚集行为,并直接测量表面过量和表面化学计量。吉布斯方程与数据的成功拟合证实了这些系统中的表面基本平衡。
更新日期:2020-07-16
中文翻译:

抗衡离子冷凝,吉布斯方程和表面活性剂结合:聚电解质及其与表面活性剂在空气-水界面处的混合物的综合描述。
通过将Gibbs方程应用于聚电解质-表面活性剂(PE-S)系统中的空气-水(A-W)界面的整体结合等温线和表面组成,我们证明了它们的表面行为可以用四种浓度半定量地解释。区域,我们将其标记为A,B,C和D。在最低浓度范围A中,本体中没有键合的PE–S配合物,但表面可能吸附有PE–S配合物。当在该区域中发生大量吸附时,表面张力(ST)会像简单的表面活性剂溶液一样随着浓度的增加而下降。B区从大量PE-S结合开始到合作结合结束,其中表面活性剂活性随合作结合的缓慢变化意味着ST的变化相对较小,尽管吸附可能很明显。这导致近似的平稳期,可能处于高或低的ST。C区开始于本体复合物中的结合失去合作性,导致表面活性剂活性随总浓度快速变化。结合大量吸附,通常会导致ST急剧下降。D区是大量PE-S复合物发生沉淀和再溶解的地方。由于溶液配合物的损失与优选表面化学计量的值相匹配,似乎在每个系统中都有明确定义的值,因此在D区可能会出现ST峰。该分析适用于实验系统,聚苯乙烯磺酸钠–烷基三甲基溴化铵和聚(二烯丙基二甲基氯)–烷基硫酸钠,有或没有添加电解质,并包括本体结合等温线,相图,聚集行为,并直接测量表面过量和表面化学计量。吉布斯方程与数据的成功拟合证实了这些系统中的表面基本平衡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号