当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of Hydrogen Bond Donor Identity and Intentional Water Addition on the Properties of Gelatin-Supported Deep Eutectic Solvent Gels.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jpcb.0c03361 Rachel E Owyeung 1, 2 , Sameer R Sonkusale 2, 3 , Matthew J Panzer 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jpcb.0c03361 Rachel E Owyeung 1, 2 , Sameer R Sonkusale 2, 3 , Matthew J Panzer 1
Affiliation
|
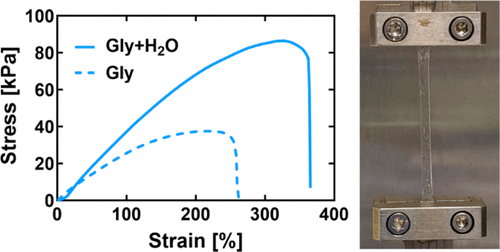
Deep eutectic solvent (DES) gel electrolytes have recently emerged as promising alternatives to ionic liquid- or water-based gels for “ionic skin” sensor applications. Researchers have also been exploring the effects that varying amounts of water may have on the local hydrogen bonding environment within a few model DES systems. In this study, the physical properties and ionic conductivities of biopolymer (gelatin)-supported gels featuring two established DESs and three DES/water mixture formulations are investigated and compared. The DES/water mixtures are formed by combining choline chloride with one of three organic hydrogen bond donors (HBDs), ethylene glycol, glycerol, or 1,2-propanediol, in a 1:2 molar ratio, together with a controlled amount of water, 25 mol % (approximately 5–6 wt % water). For the same fixed gelatin content (20 wt %), DES/water mixture gel Young’s modulus values are found to be tunable based on the organic HBD identity, increasing 6-fold from 7 (1,2-propanediol) to 42 (glycerol) kPa. Furthermore, large differences are observed in the resulting gel properties when water has been intentionally added to well-studied DESs. Coformulation with water is found to increase ethylene glycol-based DES gel toughness, measured via tensile testing, from 23 to 68 kJ/m3 while simultaneously boosting gel room temperature ionic conductivity from 3.3 to 5.2 mS/cm. These results highlight the multiple roles that controlled amounts of water in DES can play within gelatin-supported DES/mixture gel electrolytes, such as influencing gelatin self-assembly and reducing local viscosity to promote facile ion transport.
中文翻译:

氢键供体的身份和有意加水对明胶负载型深共晶溶剂凝胶性能的影响。
在“离子皮肤”传感器应用中,深共熔溶剂(DES)凝胶电解质已成为离子液体或水基凝胶的有前途的替代品。研究人员还一直在探索一些模型DES系统中不同量的水可能对局部氢键环境产生的影响。在这项研究中,研究并比较了具有两种已建立的DES和三种DES /水混合物配方的生物聚合物(明胶)负载的凝胶的物理性质和离子电导率。DES /水混合物是通过将氯化胆碱与三种有机氢键供体(HBD),乙二醇,甘油或1,2-丙二醇中的一种以1:2摩尔比结合在一起以及控制量的水形成的25摩尔%(约5-6重量%的水)。对于相同的固定明胶含量(20 wt%),发现DES /水混合物凝胶的杨氏模量值可根据有机HBD身份进行调整,从7(1,2-丙二醇)kPa增加6倍至42(甘油)kPa。此外,当将水有意添加到经过充分研究的DES中时,观察到的凝胶性质存在很大差异。与水共配制可将基于乙二醇的DES凝胶经拉伸测试测得的韧性从23 kJ / m提高至68 kJ / m3,同时从3.3到5.2毫秒/厘米升压凝胶室温离子电导率。这些结果突显了控制DES中的水量可以在明胶负载的DES /混合物凝胶电解质中发挥多种作用,例如影响明胶的自组装和降低局部粘度以促进便捷的离子迁移。
更新日期:2020-07-16
中文翻译:

氢键供体的身份和有意加水对明胶负载型深共晶溶剂凝胶性能的影响。
在“离子皮肤”传感器应用中,深共熔溶剂(DES)凝胶电解质已成为离子液体或水基凝胶的有前途的替代品。研究人员还一直在探索一些模型DES系统中不同量的水可能对局部氢键环境产生的影响。在这项研究中,研究并比较了具有两种已建立的DES和三种DES /水混合物配方的生物聚合物(明胶)负载的凝胶的物理性质和离子电导率。DES /水混合物是通过将氯化胆碱与三种有机氢键供体(HBD),乙二醇,甘油或1,2-丙二醇中的一种以1:2摩尔比结合在一起以及控制量的水形成的25摩尔%(约5-6重量%的水)。对于相同的固定明胶含量(20 wt%),发现DES /水混合物凝胶的杨氏模量值可根据有机HBD身份进行调整,从7(1,2-丙二醇)kPa增加6倍至42(甘油)kPa。此外,当将水有意添加到经过充分研究的DES中时,观察到的凝胶性质存在很大差异。与水共配制可将基于乙二醇的DES凝胶经拉伸测试测得的韧性从23 kJ / m提高至68 kJ / m3,同时从3.3到5.2毫秒/厘米升压凝胶室温离子电导率。这些结果突显了控制DES中的水量可以在明胶负载的DES /混合物凝胶电解质中发挥多种作用,例如影响明胶的自组装和降低局部粘度以促进便捷的离子迁移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号