当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural, Electronic, and Catalytic Modulation of Chelating Pyridylideneamide Ruthenium(II) Complexes
Organometallics ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.organomet.0c00205 Miquel Navarro 1 , Candela Segarra 1 , Tim Pfister 1 , Martin Albrecht 1
Organometallics ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.organomet.0c00205 Miquel Navarro 1 , Candela Segarra 1 , Tim Pfister 1 , Martin Albrecht 1
Affiliation
|
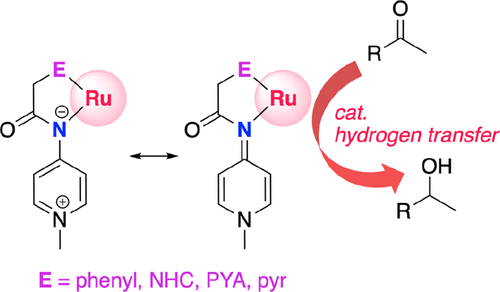
A family of Ru(cym)-type complexes bearing different pyridylideneamide (PYA) ligands has been prepared. Incorporation of diverse potentially coordinating sites afforded a series of different chelating hybrid ligands containing a PYA nitrogen as one donor site and a second variable donor site constituted of a cyclometalated aryl ring (complex 3a), a pyridine (3b), a pyridylidene (3c), another PYA unit (3d), or a triazolylidene ligand (3e). Structural and electrochemical analyses indicate considerable electronic variation in this series with decreasing donor ability from phenyl > PYA ≈ triazolylidene ≈ pyridylidene > pyridine. This trend allows the electronic properties of the metal center to be tailored and reveals the strong donor properties of PYA ligands, surpassing those of pyridine-derived N-heterocyclic carbenes. The effect of these tunable donor properties was demonstrated in transfer hydrogenation catalysis, for which a direct correlation between donor properties and catalytic activity was established.
中文翻译:

螯合亚吡啶基钌(II)配合物的结构,电子和催化调节
已经制备了带有不同吡啶基亚酰胺(PYA)配体的Ru(cym)型复合物家族。纳入各种潜在的协调位点提供了一系列不同的螯合杂配体,包含PYA氮作为一个供体位和第二个可变的供体部位,其由环金属化的芳环(络合物3a),吡啶(3b),吡啶(3c)组成。 ,另一个PYA单元(3d)或三唑基亚配体(3e)。结构和电化学分析表明,该系列中大量电子变化,其供体能力从苯基> PYA≈三唑亚基≈吡啶亚基>吡啶而降低。这种趋势允许定制金属中心的电子性能,并揭示了PYA配体的强大供体性能,超过了吡啶衍生的N-杂环卡宾。在转移氢化催化中证明了这些可调的供体性质的影响,为此建立了供体性质与催化活性之间的直接关系。
更新日期:2020-07-13
中文翻译:

螯合亚吡啶基钌(II)配合物的结构,电子和催化调节
已经制备了带有不同吡啶基亚酰胺(PYA)配体的Ru(cym)型复合物家族。纳入各种潜在的协调位点提供了一系列不同的螯合杂配体,包含PYA氮作为一个供体位和第二个可变的供体部位,其由环金属化的芳环(络合物3a),吡啶(3b),吡啶(3c)组成。 ,另一个PYA单元(3d)或三唑基亚配体(3e)。结构和电化学分析表明,该系列中大量电子变化,其供体能力从苯基> PYA≈三唑亚基≈吡啶亚基>吡啶而降低。这种趋势允许定制金属中心的电子性能,并揭示了PYA配体的强大供体性能,超过了吡啶衍生的N-杂环卡宾。在转移氢化催化中证明了这些可调的供体性质的影响,为此建立了供体性质与催化活性之间的直接关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号