当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Brønsted Base-Catalyzed Transformation of α,β-Epoxyketones Utilizing [1,2]-Phospha-Brook Rearrangement for the Synthesis of Allylic Alcohols Having a Tetrasubstituted Alkene Moiety.
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01765 Azusa Kondoh 1 , Naoko Tasato 2 , Takuma Aoki 2 , Masahiro Terada 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.orglett.0c01765 Azusa Kondoh 1 , Naoko Tasato 2 , Takuma Aoki 2 , Masahiro Terada 2
Affiliation
|
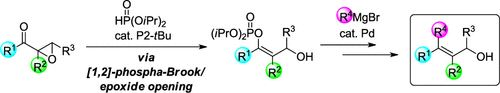
A stereoselective transformation of α,β-epoxyketones into alkenylphosphates having a hydroxymethyl group on the β-carbon was established by utilizing the [1,2]-phospha-Brook rearrangement under Brønsted base catalysis. The reaction involves the catalytic generation of an α-oxygenated carbanion located at the α-position of an epoxide moiety through the [1,2]-phospha-Brook rearrangement and the following epoxide opening. Further transformation of the alkenylphosphates by the palladium-catalyzed cross-coupling reaction with Grignard reagents provided allylic alcohols having a stereodefined all-carbon tetrasubstituted alkene moiety.
中文翻译:

布朗斯台德碱催化的α,β-环氧酮的转化利用[1,2]-磷酸-布鲁克重排合成具有四取代烯烃部分的烯丙醇。
通过在布朗斯台德碱催化下利用[1,2]-磷-布鲁克重排,将α,β-环氧酮立体选择性转化为在β-碳上具有羟甲基的烯基磷酸酯。该反应包括通过[1,2]-磷-布鲁克重排和随后的环氧化物开环催化生成位于环氧化物部分的α-位的α-氧化的碳负离子。通过与格氏试剂的钯催化的交叉偶联反应进一步转化烯基磷酸酯,提供了具有立体定义的全碳四取代烯烃部分的烯丙基醇。
更新日期:2020-07-02
中文翻译:

布朗斯台德碱催化的α,β-环氧酮的转化利用[1,2]-磷酸-布鲁克重排合成具有四取代烯烃部分的烯丙醇。
通过在布朗斯台德碱催化下利用[1,2]-磷-布鲁克重排,将α,β-环氧酮立体选择性转化为在β-碳上具有羟甲基的烯基磷酸酯。该反应包括通过[1,2]-磷-布鲁克重排和随后的环氧化物开环催化生成位于环氧化物部分的α-位的α-氧化的碳负离子。通过与格氏试剂的钯催化的交叉偶联反应进一步转化烯基磷酸酯,提供了具有立体定义的全碳四取代烯烃部分的烯丙基醇。


























 京公网安备 11010802027423号
京公网安备 11010802027423号