Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.3 ) Pub Date : 2020-06-17 , DOI: 10.1016/j.jphotochem.2020.112706 Adem Sarilmaz , Eminegul Genc , Emre Aslan , Abdurrahman Ozen , Gizem Yanalak , Faruk Ozel , Imren Hatay Patir
|
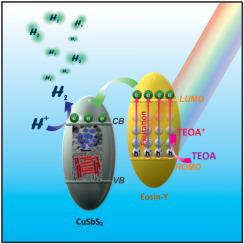
Chalcostibite (CuSbS2) semiconductor catalyst has been intensively preferred towards energy conversion processes because of its suitable bandgap, high absorption coefficient and photocatalytic activity. Herein, the photocatalytic hydrogen evolution reaction (HER) was carried out by using different shapes (Rod (R) and Dot (D)) of copper-antimony sulfides. The hydrogen evolution activities of R-CuSbS2 and d-CuSbS2 were investigated under the visible light irradiation by using eosin-Y (EY) dye and triethanolamine (TEOA) as photosensitizer and an electron donor, respectively. The photocatalytic HER rates of R-CuSbS2 and d-CuSbS2 were determined as 2140 μmolg−1 h−1 and 825 μmolg−1 h−1, respectively. R-CuSbS2 displayed higher photocatalytic activity in comparison with d-CuSbS2. The results of photocatalytic activity belonging to chalcostibite catalysts could be explained by efficient charge separation efficiency of 1D microrod materials.
中文翻译:

不同形状的CuSbS 2通过太阳能驱动的水分解产生光催化氢
黄铜矿(CuSbS 2)半导体催化剂因其合适的带隙,高吸收系数和光催化活性而在能量转换过程中得到了广泛的青睐。在此,通过使用不同形状的铜-锑硫化物(Rod(R)和Dot(D))进行光催化氢释放反应(HER)。R-CuSbS的析氢活动2和d -CuSbS 2通过使用曙红Y(EY)染料和三乙醇胺(TEOA)作为光敏剂分别和电子给体,可见光照射下研究。R-CuSbS的光催化HER率2和d -CuSbS 2测定为2140μmolg-1 h -1和825μmolg -1 h -1。与d -CuSbS 2相比,R-CuSbS 2显示出更高的光催化活性。一维微棒材料的有效电荷分离效率可以解释属于黄铜矿催化剂的光催化活性的结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号