当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile synthesis of a well-defined heteroatom-containing main chain polycarbonate for activated intracellular drug release
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-16 , DOI: 10.1039/c9qm00778d Chuanhao Sun 1, 2, 3, 4, 5 , Jiahao Wang 1, 2, 3, 4, 5 , Jieni Hu 1, 2, 3, 4, 5 , Wei Lu 5, 6, 7, 8, 9 , Zhongchen Song 5, 10, 11, 12, 13 , Yan Zhang 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-16 , DOI: 10.1039/c9qm00778d Chuanhao Sun 1, 2, 3, 4, 5 , Jiahao Wang 1, 2, 3, 4, 5 , Jieni Hu 1, 2, 3, 4, 5 , Wei Lu 5, 6, 7, 8, 9 , Zhongchen Song 5, 10, 11, 12, 13 , Yan Zhang 1, 2, 3, 4, 5
Affiliation
|
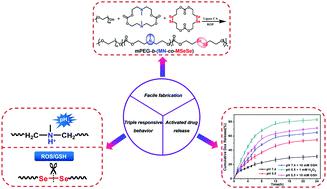
Multi-responsive drug carriers have great advantages in the targeted delivery of drugs in heterogeneous and complicated biological micro-environments. However, the sophisticated fabrication process is a great obstacle for their further application. Herein, ROS/GSH/pH triple responsive polycarbonate was rationally synthesized via the one-pot ring-opening copolymerization of the diselenide and tertiary amine macrocyclic carbonate monomers with methoxy poly(ethylene glycol) as the initiator. The micellar nanoparticles were formed by the amphiphilic block copolymers composed of a PEG segment and diselenide and tertiary amine containing the polycarbonate block via self-assembly. The diselenide and tertiary amine groups allowed for rich ROS/GSH/pH responsiveness, which caused these nanoparticles to undergo changes in the size and morphology. These micellar nanoparticles exhibited a stimuli-responsive drug release profile upon the stimulation of acidic pH, GSH, and ROS, and the release mechanism was elucidated. The blank nanoparticles were cyto-compatible, whereas the triple responsive drug-loaded nanoparticles exhibited an improved concentration-dependent and synergistic cytotoxicity towards the A549 cells. More importantly, the micellar nanoparticles could facilitate cell uptake and realize controlled intracellular release. Thus, the newly developed micellar nanocarriers could open a new avenue for smart antitumor drug delivery applications.
中文翻译:

轻松合成明确定义的含杂原子的主链聚碳酸酯,以激活细胞内药物释放
多反应药物载体在异质和复杂的生物微环境中靶向递送药物方面具有巨大优势。然而,复杂的制造工艺是其进一步应用的巨大障碍。在此,通过二硒化物和叔胺大环碳酸酯单体与甲氧基聚乙二醇作为引发剂的一锅开环共聚,合理地合成了ROS / GSH / pH三响应聚碳酸酯。胶束纳米颗粒由PEG链段和二硒化物和含有聚碳酸酯嵌段叔胺组成的两亲性嵌段共聚物形成的通过自组装。二硒化物和叔胺基团允许丰富的ROS / GSH / pH反应性,这导致这些纳米颗粒的尺寸和形态发生变化。这些胶束纳米颗粒在酸性pH,GSH和ROS刺激下表现出刺激反应性药物释放曲线,并阐明了释放机理。空白纳米颗粒具有细胞相容性,而三重响应载药纳米颗粒对A549细胞表现出改善的浓度依赖性和协同细胞毒性。更重要的是,胶束纳米颗粒可以促进细胞摄取并实现受控的细胞内释放。因此,新开发的胶束纳米载体可以为智能抗肿瘤药物递送应用开辟新途径。
更新日期:2020-07-30
中文翻译:

轻松合成明确定义的含杂原子的主链聚碳酸酯,以激活细胞内药物释放
多反应药物载体在异质和复杂的生物微环境中靶向递送药物方面具有巨大优势。然而,复杂的制造工艺是其进一步应用的巨大障碍。在此,通过二硒化物和叔胺大环碳酸酯单体与甲氧基聚乙二醇作为引发剂的一锅开环共聚,合理地合成了ROS / GSH / pH三响应聚碳酸酯。胶束纳米颗粒由PEG链段和二硒化物和含有聚碳酸酯嵌段叔胺组成的两亲性嵌段共聚物形成的通过自组装。二硒化物和叔胺基团允许丰富的ROS / GSH / pH反应性,这导致这些纳米颗粒的尺寸和形态发生变化。这些胶束纳米颗粒在酸性pH,GSH和ROS刺激下表现出刺激反应性药物释放曲线,并阐明了释放机理。空白纳米颗粒具有细胞相容性,而三重响应载药纳米颗粒对A549细胞表现出改善的浓度依赖性和协同细胞毒性。更重要的是,胶束纳米颗粒可以促进细胞摄取并实现受控的细胞内释放。因此,新开发的胶束纳米载体可以为智能抗肿瘤药物递送应用开辟新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号