当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the behavior of nonanoic acid and its conjugate base at the air/water interface through a combined experimental and theoretical approach
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-15 , DOI: 10.1039/d0sc02354j Man Luo 1, 2, 3, 4 , Nicholas A. Wauer 1, 2, 3, 4 , Kyle J. Angle 1, 2, 3, 4 , Abigail C. Dommer 1, 2, 3, 4 , Meishi Song 1, 2, 3, 4 , Christopher M. Nowak 1, 2, 3, 4 , Rommie E. Amaro 1, 2, 3, 4 , Vicki H. Grassian 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-06-15 , DOI: 10.1039/d0sc02354j Man Luo 1, 2, 3, 4 , Nicholas A. Wauer 1, 2, 3, 4 , Kyle J. Angle 1, 2, 3, 4 , Abigail C. Dommer 1, 2, 3, 4 , Meishi Song 1, 2, 3, 4 , Christopher M. Nowak 1, 2, 3, 4 , Rommie E. Amaro 1, 2, 3, 4 , Vicki H. Grassian 1, 2, 3, 4, 5
Affiliation
|
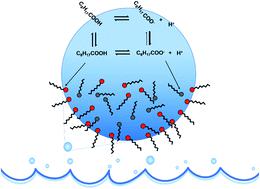
The partitioning of medium-chain fatty acid surfactants such as nonanoic acid (NA) between the bulk phase and the air/water interface is of interest to a number of fields including marine and atmospheric chemistry. However, questions remain about the behavior of these molecules, the contributions of various relevant chemical equilibria, and the impact of pH, salt and bulk surfactant concentrations. In this study, the surface adsorption of nonanoic acid and its conjugate base is quantitatively investigated at various pH values, surfactant concentrations and the presence of salts. Surface concentrations of protonated and deprotonated species are dictated by surface-bulk equilibria which can be calculated from thermodynamic considerations. Notably we conclude that the surface dissociation constant of soluble surfactants cannot be directly obtained from these experimental measurements, however, we show that molecular dynamics (MD) simulation methods, such as free energy perturbation (FEP), can be used to calculate the surface acid dissociation constant relative to that in the bulk. These simulations show that nonanoic acid is less acidic at the surface compared to in the bulk solution with a pKa shift of 1.1 ± 0.6, yielding a predicted surface pKa of 5.9 ± 0.6. A thermodynamic cycle for nonanoic acid and its conjugate base between the air/water interface and the bulk phase can therefore be established. Furthermore, the effect of salts, namely NaCl, on the surface activity of protonated and deprotonated forms of nonanoic acid is also examined. Interestingly, salts cause both a decrease in the bulk pKa of nonanoic acid and a stabilization of both the protonated and deprotonated forms at the surface. Overall, these results suggest that the deprotonated medium-chain fatty acids under ocean conditions can also be present within the sea surface microlayer (SSML) present at the ocean/atmosphere interface due to the stabilization effect of the salts in the ocean. This allows the transfer of these species into sea spray aerosols (SSAs). More generally, we present a framework with which the behavior of partially soluble species at the air/water interface can be predicted from surface adsorption models and the surface pKa can be predicted from MD simulations.
中文翻译:

通过组合的实验和理论方法洞察壬酸及其共轭碱在空气/水界面的行为
中链脂肪酸表面活性剂(如壬酸(NA))在本体相与空气/水界面之间的分配对包括海洋和大气化学在内的许多领域都非常重要。但是,关于这些分子的行为,各种相关化学平衡的贡献以及pH,盐和表面活性剂浓度的影响,仍然存在疑问。在这项研究中,在各种pH值,表面活性剂浓度和盐的存在下,对壬酸及其共轭碱的表面吸附进行了定量研究。质子化和去质子化物质的表面浓度由表面总体平衡决定,该平衡可从热力学考虑因素计算得出。值得注意的是,我们得出的结论是,不能直接从这些实验测量值中获得可溶性表面活性剂的表面解离常数,但是,我们证明了分子动力学(MD)模拟方法,例如自由能扰动(FEP),可以用于计算表面酸相对于本体的解离常数。这些模拟表明,壬酸在表面上的酸性比在使用ap的本体溶液中低。K a偏移为1.1±0.6,得出的预测表面p K a为5.9±0.6。因此,可以建立壬酸及其在空气/水界面与本体相之间的共轭碱的热力学循环。此外,还检查了盐即NaCl对壬酸的质子化和去质子化形式的表面活性的影响。有趣的是,盐会导致体积p K a均减小。壬酸在表面上的质子化和去质子化形式的稳定化。总体而言,这些结果表明,由于海洋中盐类的稳定作用,在海洋条件下去质子化的中链脂肪酸也可能存在于海洋/大气界面处的海面微层(SSML)中。这允许将这些物种转移到海雾气溶胶(SSA)中。更一般而言,我们提出了一个框架,利用该框架可以从表面吸附模型预测空气/水界面处的部分可溶物质的行为,并可以从MD模拟预测表面p K a。
更新日期:2020-06-15
中文翻译:

通过组合的实验和理论方法洞察壬酸及其共轭碱在空气/水界面的行为
中链脂肪酸表面活性剂(如壬酸(NA))在本体相与空气/水界面之间的分配对包括海洋和大气化学在内的许多领域都非常重要。但是,关于这些分子的行为,各种相关化学平衡的贡献以及pH,盐和表面活性剂浓度的影响,仍然存在疑问。在这项研究中,在各种pH值,表面活性剂浓度和盐的存在下,对壬酸及其共轭碱的表面吸附进行了定量研究。质子化和去质子化物质的表面浓度由表面总体平衡决定,该平衡可从热力学考虑因素计算得出。值得注意的是,我们得出的结论是,不能直接从这些实验测量值中获得可溶性表面活性剂的表面解离常数,但是,我们证明了分子动力学(MD)模拟方法,例如自由能扰动(FEP),可以用于计算表面酸相对于本体的解离常数。这些模拟表明,壬酸在表面上的酸性比在使用ap的本体溶液中低。K a偏移为1.1±0.6,得出的预测表面p K a为5.9±0.6。因此,可以建立壬酸及其在空气/水界面与本体相之间的共轭碱的热力学循环。此外,还检查了盐即NaCl对壬酸的质子化和去质子化形式的表面活性的影响。有趣的是,盐会导致体积p K a均减小。壬酸在表面上的质子化和去质子化形式的稳定化。总体而言,这些结果表明,由于海洋中盐类的稳定作用,在海洋条件下去质子化的中链脂肪酸也可能存在于海洋/大气界面处的海面微层(SSML)中。这允许将这些物种转移到海雾气溶胶(SSA)中。更一般而言,我们提出了一个框架,利用该框架可以从表面吸附模型预测空气/水界面处的部分可溶物质的行为,并可以从MD模拟预测表面p K a。



























 京公网安备 11010802027423号
京公网安备 11010802027423号