当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Stereocontrolled Formal [3 + 2]-Cycloaddition of CO2 for the Synthesis of Enantiopure Spiro[indoline-3,5'-oxazolidine]-2,2'-diones under Aqueous and Ambient Conditions.
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.orglett.0c01526 Saumen Hajra 1 , Anurag Biswas 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.orglett.0c01526 Saumen Hajra 1 , Anurag Biswas 1
Affiliation
|
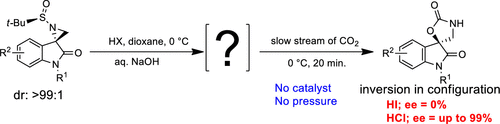
A highly efficient regio- and stereoselective spontaneous formal [3 + 2]-cycloaddition of CO2 in aqueous medium is developed for the one-pot synthesis of spiro[indoline-3,5′-oxazolidine]-2,2′-diones with excellent enantiopuirity (ee up to 99%) under catalyst-free and ambient conditions. The detailed study reveals NH-spiroaziridine- and 3-(aminomethyl)-3-chloro-oxindoles, two in situ generated reactive intermediate compounds for the spontaneous cycloaddition with CO2, and the latter is responsible for the stereoselectivity. An unprecedented mechanism of desulfinylation is also disclosed herewith.
中文翻译:

在水性和常温条件下,无催化剂的立体控制CO2的正式[3 + 2]-环加成反应,用于合成对映纯的螺[吲哚啉-3,5'-恶唑烷] -2,2'-二酮。
开发了一种高效的在水介质中对CO 2进行区域选择性和立体选择性的自发形式[3 + 2]环加成反应,用于一锅合成螺[吲哚啉-3,5'-恶唑烷] -2,2'-二酮与在无催化剂和环境条件下具有优异的对映体纯度(ee高达99%)。详细的研究揭示了NH-螺氮丙啶和3-(氨基甲基)-3-氯-氧吲哚,这两种原位生成的反应性中间化合物,可与CO 2自发环加成,后者负责立体选择性。与此同时,也公开了前所未有的脱亚磺酰化机理。
更新日期:2020-07-02
中文翻译:

在水性和常温条件下,无催化剂的立体控制CO2的正式[3 + 2]-环加成反应,用于合成对映纯的螺[吲哚啉-3,5'-恶唑烷] -2,2'-二酮。
开发了一种高效的在水介质中对CO 2进行区域选择性和立体选择性的自发形式[3 + 2]环加成反应,用于一锅合成螺[吲哚啉-3,5'-恶唑烷] -2,2'-二酮与在无催化剂和环境条件下具有优异的对映体纯度(ee高达99%)。详细的研究揭示了NH-螺氮丙啶和3-(氨基甲基)-3-氯-氧吲哚,这两种原位生成的反应性中间化合物,可与CO 2自发环加成,后者负责立体选择性。与此同时,也公开了前所未有的脱亚磺酰化机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号