当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Bioisosteric Substituents by a Deep Neural Network.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.jcim.0c00290 Peter Ertl 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.jcim.0c00290 Peter Ertl 1
Affiliation
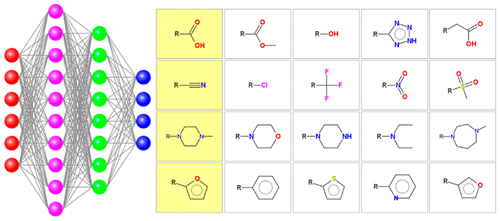
|
Bioisosteric design is a classical technique used in medicinal chemistry to improve potency, druglike properties, or the synthetic accessibility of a compound or to find similar potent compounds that exist in novel chemical space. Bioisosteric design involves replacing part of a molecule by another part that has similar properties. Such replacements may be identified by applying medicinal chemistry knowledge, by mining chemical databases or by choosing analogues similar in molecular physicochemical properties. In this article, a novel approach to identify bioisosteric analogues is described where the suggestions are made by a deep neural network trained on data collected from a large corpus of medicinal chemistry literature. The network trained in this way is able to mimic the decision making of experienced medicinal chemists and identify standard as well as nonclassical bioisosteric analogues, even for the structures outside the training set. Examples of the results are provided and application possibilities are discussed.
中文翻译:

通过深层神经网络识别生物等位取代基。
生物立体异构设计是用于药物化学的经典技术,可提高化合物的效价,类药物性质或合成可及性,或发现存在于新化学空间中的相似有效化合物。生物立体设计涉及将分子的一部分替换为具有相似特性的另一部分。可以通过应用药物化学知识,通过挖掘化学数据库或通过选择分子物理化学性质相似的类似物来识别此类替代物。在本文中,介绍了一种识别生物立体异构体类似物的新颖方法,其中的建议是通过对从大量药物化学文献中收集的数据进行训练的深度神经网络提出的。以这种方式训练的网络能够模仿经验丰富的药物化学家的决策,甚至可以针对训练集外的结构识别标准以及非经典的生物立体异构体。提供了结果示例,并讨论了应用可能性。
更新日期:2020-07-27
中文翻译:

通过深层神经网络识别生物等位取代基。
生物立体异构设计是用于药物化学的经典技术,可提高化合物的效价,类药物性质或合成可及性,或发现存在于新化学空间中的相似有效化合物。生物立体设计涉及将分子的一部分替换为具有相似特性的另一部分。可以通过应用药物化学知识,通过挖掘化学数据库或通过选择分子物理化学性质相似的类似物来识别此类替代物。在本文中,介绍了一种识别生物立体异构体类似物的新颖方法,其中的建议是通过对从大量药物化学文献中收集的数据进行训练的深度神经网络提出的。以这种方式训练的网络能够模仿经验丰富的药物化学家的决策,甚至可以针对训练集外的结构识别标准以及非经典的生物立体异构体。提供了结果示例,并讨论了应用可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号