当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Change in Domain Cooperativity Drives the Function of Calnuc.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00207 Ravichandran Vignesh 1 , Gopala Krishna Aradhyam 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.biochem.0c00207 Ravichandran Vignesh 1 , Gopala Krishna Aradhyam 1
Affiliation
|
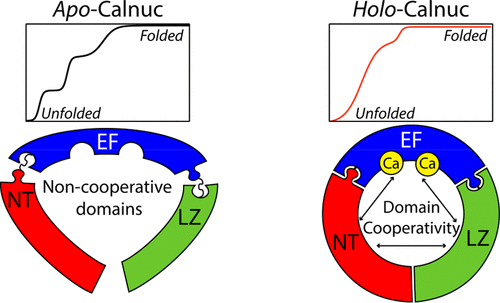
With the increasing incidence of neurodegenerative disorders, there is an urgent need to understand the protein folding process. Examining the folding process of multidomain proteins remains a prime challenge, as their complex conformational dynamics make them highly susceptible to misfolding and/or aggregation. The presence of multiple domains in a protein can lead to interaction between the partially folded domains, thereby driving misfolding and/or aggregation. Calnuc is one such multidomain protein for which Ca2+ binding plays a pivotal role in governing its structural dynamics and stability and, presumably, in directing its interactions with other proteins. We demonstrate differential structural dynamics between the Ca2+-free and Ca2+-bound forms of calnuc. In the absence of Ca2+, full-length calnuc displays equilibrium structural transitions with four intermediate states, reporting a sum of the behavioral properties of its individual domains. Fragment-based studies illustrate the sequential events of structure adoption proceeding in the following order: EF domain followed by the NT and LZ domains in the apo state. On the other hand, Ca2+ binding increases domain cooperativity and enables the protein to fold as a single unit. Single-tryptophan mutant proteins, designed in a domain-dependent manner, confirm an increase in the number of interdomain interactions in the Ca2+-bound form as compared to the Ca2+-free state of the protein, thereby providing insight into its folding process. The attenuated domain crosstalk in apo-calnuc is likely to influence and regulate its physiologically important intermolecular interactions.
中文翻译:

域合作性的变化驱动了Calnuc的功能。
随着神经退行性疾病发生率的增加,迫切需要了解蛋白质折叠过程。检查多结构域蛋白的折叠过程仍然是一个主要挑战,因为它们复杂的构象动力学使它们高度容易发生错误折叠和/或聚集。蛋白质中多个结构域的存在可导致部分折叠的结构域之间发生相互作用,从而驱动错误折叠和/或聚集。钙调蛋白是一种这样的多结构域蛋白,Ca 2+的结合在控制其结构动力学和稳定性以及推测其与其他蛋白的相互作用中起着关键作用。我们展示了无Ca 2+和Ca 2+之间的差异结构动力学钙蛋白的束缚形式。在没有Ca 2+的情况下,全长钙粉显示出具有四个中间状态的平衡结构转变,报告了其各个域的行为特性之和。基于片段的研究说明了结构采用的顺序事件,其顺序如下:EF域,然后是apo状态的NT和LZ域。另一方面,Ca 2+的结合增加了域的协同作用,并使蛋白质折叠成一个单元。单色氨酸突变蛋白,以依赖于域的方式设计,在确认的域间在CA交互的数量的增加2+结合形式相比,钙2+蛋白质的游离状态,从而提供对其折叠过程的洞察力。载脂蛋白钙蛋白中的减弱的域串扰可能影响并调节其生理学上重要的分子间相互作用。
更新日期:2020-07-14
中文翻译:

域合作性的变化驱动了Calnuc的功能。
随着神经退行性疾病发生率的增加,迫切需要了解蛋白质折叠过程。检查多结构域蛋白的折叠过程仍然是一个主要挑战,因为它们复杂的构象动力学使它们高度容易发生错误折叠和/或聚集。蛋白质中多个结构域的存在可导致部分折叠的结构域之间发生相互作用,从而驱动错误折叠和/或聚集。钙调蛋白是一种这样的多结构域蛋白,Ca 2+的结合在控制其结构动力学和稳定性以及推测其与其他蛋白的相互作用中起着关键作用。我们展示了无Ca 2+和Ca 2+之间的差异结构动力学钙蛋白的束缚形式。在没有Ca 2+的情况下,全长钙粉显示出具有四个中间状态的平衡结构转变,报告了其各个域的行为特性之和。基于片段的研究说明了结构采用的顺序事件,其顺序如下:EF域,然后是apo状态的NT和LZ域。另一方面,Ca 2+的结合增加了域的协同作用,并使蛋白质折叠成一个单元。单色氨酸突变蛋白,以依赖于域的方式设计,在确认的域间在CA交互的数量的增加2+结合形式相比,钙2+蛋白质的游离状态,从而提供对其折叠过程的洞察力。载脂蛋白钙蛋白中的减弱的域串扰可能影响并调节其生理学上重要的分子间相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号