当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the human NLRP9 pyrin domain reveals a bent N‐terminal loop that may regulate inflammasome assembly
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-28 , DOI: 10.1002/1873-3468.13866 Hyun Ji Ha 1 , Hyun Ho Park 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-28 , DOI: 10.1002/1873-3468.13866 Hyun Ji Ha 1 , Hyun Ho Park 1
Affiliation
|
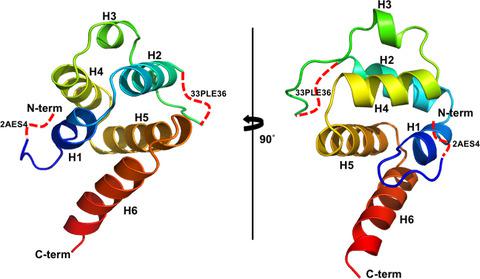
Members of the NLR family pyrin domain containing (NLRPs) are pattern recognition receptors that participate in innate immunity. They form inflammasomes, which are platforms for caspase-1 recruitment and activation. The NLRP pyrin domain (PYD) is critical for the assembly of inflammasomes due to its ability to mediate protein interactions. Despite intensive structural studies on inflammasomes with PYDs, the structure of the PYD of NLRP9-the least studied member of the family-remains unknown. Herein, we report the crystal structure of the human NLRP9 PYD at 2.1 Å resolution, which reveals a kinked N-terminal loop oriented towards the interior of the helical bundle. Based on our findings, we propose a regulatory role for the kinked N-terminal loop of NLRP9 PYD in inflammasome assembly.
中文翻译:

人 NLRP9 pyrin 结构域的晶体结构揭示了一个弯曲的 N 端环,可以调节炎症小体的组装
NLR 家族含有 pyrin 结构域 (NLRPs) 的成员是参与先天免疫的模式识别受体。它们形成炎症小体,这是 caspase-1 募集和激活的平台。NLRP pyrin 结构域 (PYD) 对炎症小体的组装至关重要,因为它具有介导蛋白质相互作用的能力。尽管对具有 PYD 的炎症小体进行了深入的结构研究,但 NLRP9 的 PYD 结构——该家族中研究最少的成员——仍然未知。在这里,我们报告了人类 NLRP9 PYD 的晶体结构,分辨率为 2.1 Å,揭示了一个朝向螺旋束内部的扭结 N 端环。基于我们的发现,我们提出了 NLRP9 PYD 的扭结 N 端环在炎性体组装中的调节作用。
更新日期:2020-06-28
中文翻译:

人 NLRP9 pyrin 结构域的晶体结构揭示了一个弯曲的 N 端环,可以调节炎症小体的组装
NLR 家族含有 pyrin 结构域 (NLRPs) 的成员是参与先天免疫的模式识别受体。它们形成炎症小体,这是 caspase-1 募集和激活的平台。NLRP pyrin 结构域 (PYD) 对炎症小体的组装至关重要,因为它具有介导蛋白质相互作用的能力。尽管对具有 PYD 的炎症小体进行了深入的结构研究,但 NLRP9 的 PYD 结构——该家族中研究最少的成员——仍然未知。在这里,我们报告了人类 NLRP9 PYD 的晶体结构,分辨率为 2.1 Å,揭示了一个朝向螺旋束内部的扭结 N 端环。基于我们的发现,我们提出了 NLRP9 PYD 的扭结 N 端环在炎性体组装中的调节作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号