Science Bulletin ( IF 18.9 ) Pub Date : 2020-06-16 , DOI: 10.1016/j.scib.2020.06.023 Haipeng Bai 1 , Tao Cheng 2 , Shangyu Li 1 , Zhenyu Zhou 1 , Hao Yang 2 , Jun Li 3 , Miao Xie 2 , Jinyu Ye 4 , Yujin Ji 2 , Youyong Li 2 , Zhiyou Zhou 4 , Shigang Sun 4 , Bo Zhang 1 , Huisheng Peng 1
|
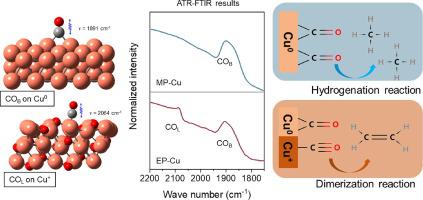
Among all CO2 electroreduction products, methane (CH4) and ethylene (C2H4) are two typical and valuable hydrocarbon products which are formed in two different pathways: hydrogenation and dimerization reactions of the same CO intermediate. Theoretical studies show that the adsorption configurations of CO intermediate determine the reaction pathways towards CH4/C2H4. However, it is challenging to experimentally control the CO adsorption configurations at the catalyst surface, and thus the hydrocarbon selectivity is still limited. Herein, we seek to synthesize two well-defined copper nanocatalysts with controllable surface structures. The two model catalysts exhibit a high hydrocarbon selectivity toward either CH4 (83%) or C2H4 (93%) under identical reduction conditions. Scanning transmission electron microscopy and X-ray absorption spectroscopy characterizations reveal the low-coordination Cu0 sites and local Cu0/Cu+ sites of the two catalysts, respectively. CO-temperature programed desorption, in-situ attenuated total reflection Fourier transform infrared spectroscopy and density functional theory studies unveil that the bridge-adsorbed CO (COB) on the low-coordination Cu0 sites is apt to be hydrogenated to CH4, whereas the bridge-adsorbed CO plus linear-adsorbed CO (COB + COL) on the local Cu0/Cu+ sites are apt to be coupled to C2H4. Our findings pave a new way to design catalysts with controllable CO adsorption configurations for high hydrocarbon product selectivity.
中文翻译:

可控的 CO 吸附决定了通过 CO2 电还原生产乙烯和甲烷
在所有CO 2电还原产物中,甲烷(CH 4)和乙烯(C 2 H 4)是两种典型且有价值的碳氢化合物产物,它们通过两种不同的途径形成:同一CO中间体的加氢和二聚反应。理论研究表明,CO中间体的吸附构型决定了CH 4 /C 2 H 4的反应途径. 然而,通过实验控制催化剂表面的 CO 吸附构型具有挑战性,因此碳氢化合物的选择性仍然受到限制。在此,我们寻求合成两种具有可控表面结构的定义明确的铜纳米催化剂。这两种模型催化剂在相同的还原条件下表现出对 CH 4 (83%) 或 C 2 H 4 (93%)的高烃选择性。扫描透射电子显微镜和 X 射线吸收光谱表征分别揭示了两种催化剂的低配位 Cu 0位点和局部 Cu 0 /Cu +位点。原位CO程序升温脱附衰减全反射傅立叶变换红外光谱和密度泛函理论研究表明,在低配位 Cu 0位点上的桥吸附 CO (CO B )易于氢化为 CH 4,而桥吸附 CO 加线性吸附局部Cu 0 /Cu +位点上的CO(CO B + CO L )易于与C 2 H 4偶联。我们的研究结果为设计具有可控 CO 吸附配置的催化剂以实现高碳氢化合物产品选择性开辟了一条新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号