Cell Chemical Biology ( IF 8.6 ) Pub Date : 2020-06-16 , DOI: 10.1016/j.chembiol.2020.05.013 Irem Avcilar-Kucukgoze 1 , Howard Gamper 2 , Christine Polte 3 , Zoya Ignatova 3 , Ralph Kraetzner 4 , Michael Shtutman 5 , Ya-Ming Hou 2 , Dawei W Dong 6 , Anna Kashina 1
|
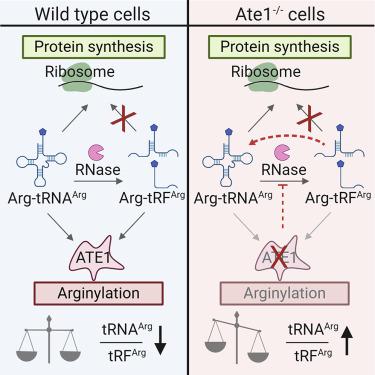
Arginyltransferase ATE1 mediates posttranslational arginylation and plays key roles in multiple physiological processes. ATE1 utilizes arginyl (Arg)-tRNAArg as the donor of Arg, putting this reaction into a direct competition with the protein synthesis machinery. Here, we address the question of ATE1- Arg-tRNAArg specificity as a potential mechanism enabling this competition in vivo. Using in vitro arginylation assays and Ate1 knockout models, we find that, in addition to full-length tRNA, ATE1 is also able to utilize short tRNAArg fragments that bear structural resemblance to tRNA-derived fragments (tRF), a recently discovered class of small regulatory non-coding RNAs with global emerging biological role. Ate1 knockout cells show a decrease in tRFArg generation and a significant increase in the ratio of tRNAArg:tRFArg compared with wild type, suggesting a functional link between tRFArg and arginylation. We propose that generation of physiologically important tRFs can serve as a switch between translation and protein arginylation.
中文翻译:

tRNAArg衍生的片段可以作为精氨酸供体用于蛋白质的精氨酸化。
精氨酸转移酶ATE1介导翻译后的精氨酰化,并在多个生理过程中发挥关键作用。ATE1利用精氨酸(Arg)-tRNA Arg作为Arg的供体,使该反应与蛋白质合成机制直接竞争。在这里,我们解决了ATE1- Arg-tRNA Arg特异性的问题,这是一种在体内实现这种竞争的潜在机制。使用体外精氨酰化测定和Ate1敲除模型,我们发现,除了全长tRNA外,ATE1还能够利用短tRNA Arg与tRNA来源的片段(tRF)具有结构相似性的片段,这是最近发现的一类具有全球新兴生物学功能的小型监管非编码RNA。与野生型相比,Ate1敲除细胞显示tRF Arg的生成减少,tRNA Arg:tRF Arg的比率显着增加,表明tRF Arg与精氨酰化之间存在功能联系。我们建议生理重要的tRF的生成可以充当翻译和蛋白质精氨酰化之间的转换。


























 京公网安备 11010802027423号
京公网安备 11010802027423号