当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
BODIPY-amino acid conjugates – tuning the optical response with a meso-heteroatom
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-15 , DOI: 10.1039/d0qo00481b Marco Farinone 1, 2, 3, 4 , Joanna Cybińska 1, 2, 3, 4 , Miłosz Pawlicki 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-15 , DOI: 10.1039/d0qo00481b Marco Farinone 1, 2, 3, 4 , Joanna Cybińska 1, 2, 3, 4 , Miłosz Pawlicki 1, 2, 3, 4
Affiliation
|
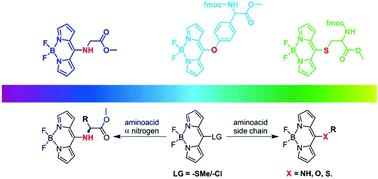
The presence of a heteroatom at the meso-position of BODIPY significantly influences the π-cloud of the main chromophore, modifying the final optical properties. In this work we obtained a series of amino acid-BODIPY hybrid skeletons with good yields through a nucleophilic aromatic substitution reaction. The heteroatom came from amino acid chains where the specific location of nitrogen, sulfur or oxygen opened a possibility of formation of a family of specific derivatives. The obtained series of hybrid materials were investigated with different spectroscopic techniques, giving insights into the specific behaviour shown by the derivatives. The observed optical responses were demonstrated to be dependent on the type of the heteroatom attached to the meso-position of the BODIPY skeleton, substantially modulating the optical response depending on the type of C–X (X = N, S, and O) interaction. Moreover, the influence of the heteroatom connected to the meso-position showed an interesting correlation in the NMR experiments (1H and 13C) and substantial differences were observed in the theoretical calculation models (DFT).
中文翻译:

BODIPY-氨基酸共轭物–用介观杂原子调节光学响应
BODIPY介观位上杂原子的存在会显着影响主发色团的π云,从而改变最终的光学性质。在这项工作中,我们通过亲核芳香族取代反应以高收率获得了一系列氨基酸-BODIPY杂种骨架。杂原子来自氨基酸链,其中氮,硫或氧的特定位置打开了形成特定衍生物家族的可能性。使用不同的光谱技术对获得的一系列杂化材料进行了研究,从而深入了解了衍生物显示的特定行为。已证明观察到的光学响应取决于附着于内消旋体的杂原子的类型BODIPY骨架的位置,根据C–X(X = N,S和O)相互作用的类型实质上调节了光学响应。此外,杂原子连接到中间位的影响在NMR实验(1 H和13 C)中显示出有趣的相关性,并且在理论计算模型(DFT)中观察到了实质性差异。
更新日期:2020-06-15
中文翻译:

BODIPY-氨基酸共轭物–用介观杂原子调节光学响应
BODIPY介观位上杂原子的存在会显着影响主发色团的π云,从而改变最终的光学性质。在这项工作中,我们通过亲核芳香族取代反应以高收率获得了一系列氨基酸-BODIPY杂种骨架。杂原子来自氨基酸链,其中氮,硫或氧的特定位置打开了形成特定衍生物家族的可能性。使用不同的光谱技术对获得的一系列杂化材料进行了研究,从而深入了解了衍生物显示的特定行为。已证明观察到的光学响应取决于附着于内消旋体的杂原子的类型BODIPY骨架的位置,根据C–X(X = N,S和O)相互作用的类型实质上调节了光学响应。此外,杂原子连接到中间位的影响在NMR实验(1 H和13 C)中显示出有趣的相关性,并且在理论计算模型(DFT)中观察到了实质性差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号