当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Terminal Rh Methylidene from Activation of CH2Cl2
Organometallics ( IF 2.8 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.organomet.0c00031 Travis J. Morrow 1 , Jordan R. Gipper 1 , William E. Christman 1 , Navamoney Arulsamy 1 , Elliott B. Hulley 1
Organometallics ( IF 2.8 ) Pub Date : 2020-06-15 , DOI: 10.1021/acs.organomet.0c00031 Travis J. Morrow 1 , Jordan R. Gipper 1 , William E. Christman 1 , Navamoney Arulsamy 1 , Elliott B. Hulley 1
Affiliation
|
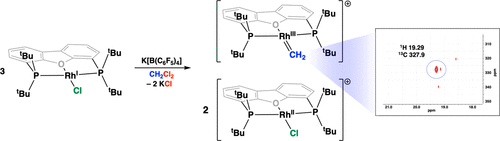
Oxidative addition of carbon–halogen bonds at transition metals typically follows either a two-electron pathway (concerted M–R/M–X formation) or a radical chain pathway (stepwise M–R/M–X formation). When the reactive metal species is generated slowly, however, both mechanisms can compete to yield unexpected reactivity paths. The present report highlights the synthesis of rhodium methylidenes from chloroalkanes (e.g., CH2Cl2 and CHCl3) at POP-pincer frameworks (e.g., POP = 4,6-bis(di-tert-butylphosphino)dibenzo[b,d]furan) via a cascade of halide abstraction and electron transfer steps. Experimental and computational studies are reported that support the proposed mechanism, including characterization of important reaction intermediates. The overall transformation represents a route toward reactive metal alkylidenes using milder and less-reactive carbenoid precursors than what is presently used.
中文翻译:

CH 2 Cl 2活化引起的末端Rh亚甲基
过渡金属上碳-卤素键的氧化加成通常遵循两条电子途径(经证实的M–R / M–X形成)或自由基链途径(逐步进行的M–R / M–X形成)。但是,当反应性金属物质缓慢生成时,两种机制都可以竞争以产生意想不到的反应路径。本报告重点介绍了在POP钳构架(例如POP = 4,6-双(二叔丁基膦基)二苯并[ b,d]下由氯代烷烃(例如CH 2 Cl 2和CHCl 3)合成亚甲基铑的方法。[呋喃])通过一系列卤化物提取和电子转移步骤来完成。据报道,实验和计算研究支持所提出的机制,包括重要反应中间体的表征。总的转化代表使用比目前使用的温和且反应性较低的类胡萝卜素前体向反应性金属亚烷基的途径。
更新日期:2020-07-13
中文翻译:

CH 2 Cl 2活化引起的末端Rh亚甲基
过渡金属上碳-卤素键的氧化加成通常遵循两条电子途径(经证实的M–R / M–X形成)或自由基链途径(逐步进行的M–R / M–X形成)。但是,当反应性金属物质缓慢生成时,两种机制都可以竞争以产生意想不到的反应路径。本报告重点介绍了在POP钳构架(例如POP = 4,6-双(二叔丁基膦基)二苯并[ b,d]下由氯代烷烃(例如CH 2 Cl 2和CHCl 3)合成亚甲基铑的方法。[呋喃])通过一系列卤化物提取和电子转移步骤来完成。据报道,实验和计算研究支持所提出的机制,包括重要反应中间体的表征。总的转化代表使用比目前使用的温和且反应性较低的类胡萝卜素前体向反应性金属亚烷基的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号