当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapour-Liquid Equilibria for the binary systems of pentafluoroethane {(R125) + 2,3,3,3-tetrafluoroprop-1-ene (R1234yf)} and {trans-1,3,3,3-tetrafluoropropene R1234ze(E)}
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106222 Tao Yang , Xiaozhen Hu , Xianyang Meng , Jiangtao Wu
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106222 Tao Yang , Xiaozhen Hu , Xianyang Meng , Jiangtao Wu
|
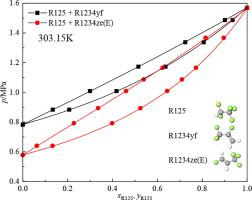
Abstract In this work, isothermal vapour-liquid equilibrium (VLE) data for the binary mixtures of pentafluoroethane (R125) + 2,3,3,3-tetrafluoroprop-1-ene (R1234yf) and trans-1,3,3,3-tetrafluoropene (R1234ze(E)) have been investigated. The experimental measurements were carried out by using the AnTLcirCapValVis analytical method over the temperature range from 283.15 K to 323.15 K. The standard uncertainties of temperature, pressure and mole fractions are 10 mK, 0.5 kPa and 0.005, respectively. The Peng-Robinson (PR) equation of state (EoS) and its modifications from Stryjek and Vera (PRSV and PRSV2) combined with Wong-Sandler (WS) mixing rule and the non-random two-liquid activity coefficient model (NRTL) were employed to correlate the parameters of binary mixtures, respectively. The PRSV2 + WS + NRTL model shows the best consistency with the experimental VLE data compared with the other two models (PR + WS + NRTL and PRSV + WS + NRTL). The average absolute relative deviation of pressure (AARD(p)) and average absolute deviation of vapour phase mole fraction (AAD(y)) are 0.10% and 0.0012 for R125 + R1234yf, 0.16% and 0.0014 for R125 + R1234ze(E), respectively. Both the experimental data and the calculated results imply that R125 + R1234yf and R125 + R1234ze(E) are zeotropic binary mixtures over the studied temperatures.
中文翻译:

五氟乙烷 {(R125) + 2,3,3,3-四氟丙烯-1-烯 (R1234yf)} 和 {trans-1,3,3,3-四氟丙烯 R1234ze(E)} 二元系统的气液平衡
摘要 在这项工作中,五氟乙烷 (R125) + 2,3,3,3-四氟丙-1-烯 (R1234yf) 和反式-1,3,3,3 二元混合物的等温汽液平衡 (VLE) 数据-四氟戊烯 (R1234ze(E)) 已被研究。实验测量使用 AnTLcirCapValVis 分析方法在 283.15 K 至 323.15 K 的温度范围内进行。温度、压力和摩尔分数的标准不确定度分别为 10 mK、0.5 kPa 和 0.005。Peng-Robinson (PR) 状态方程 (EoS) 及其修改自 Stryjek 和 Vera(PRSV 和 PRSV2)结合 Wong-Sandler (WS) 混合规则和非随机两液活度系数模型 (NRTL)分别用于关联二元混合物的参数。PRSV2 + WS + NRTL 模型与其他两个模型(PR + WS + NRTL 和 PRSV + WS + NRTL)相比,与实验 VLE 数据的一致性最好。压力的平均绝对相对偏差(AARD(p))和气相摩尔分数的平均绝对偏差(AAD(y))对于R125+R1234yf分别为0.10%和0.0012,对于R125+R1234ze(E)为0.16%和0.0014,分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。
更新日期:2020-11-01
中文翻译:

五氟乙烷 {(R125) + 2,3,3,3-四氟丙烯-1-烯 (R1234yf)} 和 {trans-1,3,3,3-四氟丙烯 R1234ze(E)} 二元系统的气液平衡
摘要 在这项工作中,五氟乙烷 (R125) + 2,3,3,3-四氟丙-1-烯 (R1234yf) 和反式-1,3,3,3 二元混合物的等温汽液平衡 (VLE) 数据-四氟戊烯 (R1234ze(E)) 已被研究。实验测量使用 AnTLcirCapValVis 分析方法在 283.15 K 至 323.15 K 的温度范围内进行。温度、压力和摩尔分数的标准不确定度分别为 10 mK、0.5 kPa 和 0.005。Peng-Robinson (PR) 状态方程 (EoS) 及其修改自 Stryjek 和 Vera(PRSV 和 PRSV2)结合 Wong-Sandler (WS) 混合规则和非随机两液活度系数模型 (NRTL)分别用于关联二元混合物的参数。PRSV2 + WS + NRTL 模型与其他两个模型(PR + WS + NRTL 和 PRSV + WS + NRTL)相比,与实验 VLE 数据的一致性最好。压力的平均绝对相对偏差(AARD(p))和气相摩尔分数的平均绝对偏差(AAD(y))对于R125+R1234yf分别为0.10%和0.0012,对于R125+R1234ze(E)为0.16%和0.0014,分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。分别。实验数据和计算结果都表明 R125 + R1234yf 和 R125 + R1234ze(E) 在研究温度下是非共沸二元混合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号