当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct electrochemical determination of very low levels of 5-hydroxymethyl furfural in natural honey by cyclic and square wave voltammetric techniques
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jelechem.2020.114326 Ines Salhi , Youssef Samet , Mahmoud Trabelsi
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jelechem.2020.114326 Ines Salhi , Youssef Samet , Mahmoud Trabelsi
|
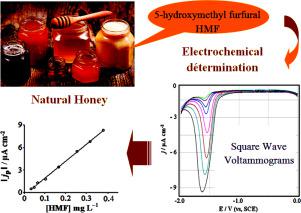
Abstract The present study reported the electrochemical determination of 5-hydroxymethylfurfural (HMF) obtained by the degradation of fructose, with a glassy carbon electrode. This study was performed by cyclic voltammetry (CV) and square wave voltammetry (SWV). The aim of this work was to propose a direct and rapid technique to quantify HMF in honey. The effect of scan rate, pH and temperature was evaluated to select the optimum experimental conditions. HMF was irreversibly reduced, thus producing a single cathodic peak at a potential of −1.6 V vsSCE in the presence of Na2SO4 as supporting electrolyte involving a transfer of two electrons. The diffusion coefficient was calculated from convoluted cyclic voltammograms and was found to be 2.23 10−6 cm2 s−1. The voltammetric determination of HMF was carried out under optimum conditions. The calibration curves for the determination of HMF exhibited the good linear responses within the concentration range from 0.15 to 1.26 mg L−1 for CV method and from 0.03 mg L−1 to 0.38 mg L−1 for SWV method. In water, the quantification limits of HMF were about 83.3 10−3 mg L−1 and 7.2 10−3 mg L−1 for CV and SWV, respectively. The SWV technique showed a higher sensitivity and reproducibility in analytical response. In honey solution, the limit of quantification was found to be 0.02 mg L−1. The method was successfully applied to the analysis of HMF in honey and syrup samples containing different concentrations with good recovery values.
中文翻译:

用循环和方波伏安法直接电化学测定天然蜂蜜中极低含量的 5-羟甲基糠醛
摘要 本研究报道了用玻碳电极电化学测定果糖降解得到的 5-羟甲基糠醛 (HMF)。本研究通过循环伏安法 (CV) 和方波伏安法 (SWV) 进行。这项工作的目的是提出一种直接快速的技术来量化蜂蜜中的 HMF。评估扫描速率、pH 值和温度的影响以选择最佳实验条件。HMF 被不可逆地还原,因此在 Na2SO4 作为支持电解质的存在下,在 -1.6 V vsSCE 的电位下产生单个阴极峰,涉及两个电子的转移。扩散系数由复杂的循环伏安图计算,结果为 2.23 10-6 cm2 s-1。HMF 的伏安法测定在最佳条件下进行。用于测定 HMF 的校准曲线在 CV 方法的 0.15 至 1.26 mg L-1 和 SWV 方法的 0.03 mg L-1 至 0.38 mg L-1 浓度范围内表现出良好的线性响应。在水中,CV 和 SWV 的 HMF 定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。对于 CV 和 SWV,HMF 的定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。对于 CV 和 SWV,HMF 的定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。
更新日期:2020-09-01
中文翻译:

用循环和方波伏安法直接电化学测定天然蜂蜜中极低含量的 5-羟甲基糠醛
摘要 本研究报道了用玻碳电极电化学测定果糖降解得到的 5-羟甲基糠醛 (HMF)。本研究通过循环伏安法 (CV) 和方波伏安法 (SWV) 进行。这项工作的目的是提出一种直接快速的技术来量化蜂蜜中的 HMF。评估扫描速率、pH 值和温度的影响以选择最佳实验条件。HMF 被不可逆地还原,因此在 Na2SO4 作为支持电解质的存在下,在 -1.6 V vsSCE 的电位下产生单个阴极峰,涉及两个电子的转移。扩散系数由复杂的循环伏安图计算,结果为 2.23 10-6 cm2 s-1。HMF 的伏安法测定在最佳条件下进行。用于测定 HMF 的校准曲线在 CV 方法的 0.15 至 1.26 mg L-1 和 SWV 方法的 0.03 mg L-1 至 0.38 mg L-1 浓度范围内表现出良好的线性响应。在水中,CV 和 SWV 的 HMF 定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。对于 CV 和 SWV,HMF 的定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。对于 CV 和 SWV,HMF 的定量限分别约为 83.3 10-3 mg L-1 和 7.2 10-3 mg L-1。SWV 技术在分析响应中显示出更高的灵敏度和重现性。在蜂蜜溶液中,发现定量限为 0.02 mg L-1。该方法成功应用于不同浓度蜂蜜和糖浆样品中 HMF 的分析,具有良好的回收率。

























 京公网安备 11010802027423号
京公网安备 11010802027423号