Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.freeradbiomed.2020.06.005 Toshiaki Teratani 1 , Kengo Tomita 2 , Sachiko Toma-Fukai 3 , Yutaro Nakamura 4 , Toshimasa Itoh 5 , Hikaru Shimizu 4 , Yasunaga Shiraishi 6 , Nao Sugihara 2 , Masaaki Higashiyama 2 , Takahiko Shimizu 7 , Ikuo Inoue 8 , Yasuhiro Takenaka 9 , Ryota Hokari 2 , Takeshi Adachi 10 , Toshiyuki Shimizu 4 , Soichiro Miura 11 , Takanori Kanai 1
|
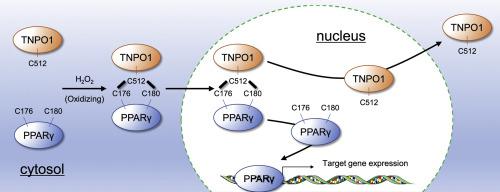
The nuclear receptor peroxisome proliferator-activated receptor (PPAR)γ has been implicated in the pathogenesis of various human diseases including fatty liver. Although nuclear translocation of PPARγ plays an important role in PPARγ signaling, details of the translocation mechanisms have not been elucidated. Here we demonstrate that PPARγ2 translocates to the nucleus and activates signal transduction through H2O2-dependent formation of a PPARγ2 and transportin (Tnpo)1 complex via redox-sensitive disulfide bonds between cysteine (Cys)176 and Cys180 of the former and Cys512 of the latter. Using hepatocyte cultures and mouse models, we show that cytosolic H2O2/Tnpo1-dependent nuclear translocation enhances the amount of DNA-bound PPARγ and downstream signaling, leading to triglyceride accumulation in hepatocytes and liver. These findings expand our understanding of the mechanism underlying the nuclear translocation of PPARγ, and suggest that the PPARγ and Tnpo1 complex and surrounding redox environment are potential therapeutic targets in the treatment of PPARγ-related diseases.
中文翻译:

氧化还原依赖性的PPARγ/ Tnpo1复合物的形成增强了PPARγ的核定位和信号传导。
核受体过氧化物酶体增殖物激活受体(PPAR)γ与多种人类疾病(包括脂肪肝)的发病机制有关。尽管PPARγ的核易位在PPARγ信号传导中起着重要作用,但尚未阐明其转位机制的细节。在这里,我们证明PPARγ2易位至细胞核,并通过半胱氨酸(Cys)176和Cys180之间的半胱氨酸(Cys)176和Cys180与Cys512之间的氧化还原敏感的二硫键,通过H 2 O 2依赖性的PPARγ2和transportin(Tnpo)1复合物激活信号传导后者。使用肝细胞培养物和小鼠模型,我们表明胞质H 2 O 2/ Tnpo1依赖性核易位增加了DNA结合的PPARγ和下游信号传导的量,导致甘油三酯在肝细胞和肝脏中积累。这些发现扩大了我们对PPARγ核易位的机制的认识,并表明PPARγ和Tnpo1复合物以及周围的氧化还原环境是治疗PPARγ相关疾病的潜在治疗靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号