Biomaterials ( IF 14.0 ) Pub Date : 2020-06-15 , DOI: 10.1016/j.biomaterials.2020.120188 Suyeon You 1 , Hyoungtai Kim 2 , Hye-Youn Jung 1 , Boram Kim 1 , Eun Jung Lee 2 , Jin Woo Kim 2 , Yoonkyung Kim 1
|
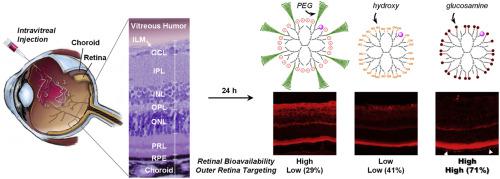
Age-related macular degeneration (AMD) is one of the leading causes of irreversible blindness, generally affecting people over 50 years of age in industrialized countries. Despite the effectiveness of anti-vascular endothelial growth factor (VEGF) therapy in attenuating the growth of new blood vessels, substantial visual improvements are rare with this complex disease. Furthermore, the current regimen of repeated monthly intravitreal injections of drugs can result in serious side effects. Combination therapies—to complement anti-VEGF alone—with a prolonged therapeutic effect and efficient delivery to the intended site are urgently needed, which could be realized through the use of carefully designed nanocarriers. To understand the physicochemical effects (e.g., size, charge, geometry) of intravitreally administered nanocarriers on their bioavailability, distribution, and targeting efficiency across multiple layers of the retina, here we prepared seven different types of surface-functionalized water-soluble dendritic nanocarriers with hydrodynamic sizes mostly under 5 nm. A similar stoichiometric amount of fluorophore was covalently attached to each of these biocompatible nanocarriers for quantitative analyses by confocal microscopy of cryosectioned healthy mouse eyes. Interestingly, at 24 h post-injection, the nanocarrier with multiple copies of glucosamine on the surface (DNSG) accumulated predominantly in the photoreceptor layer and the retinal pigment epithelium (RPE), which are speculated to be associated with AMD pathogenesis (i.e., target sites). Furthermore, extended residence at these outer retinal layers was demonstrated by DNSG, which appeared to gradually turn into micron-scale particles potentially through aggregation. Our systematic findings may provide useful guidelines for the rational design of intravitreal nanocarriers to treat vision-threatening retinal diseases, including AMD.
中文翻译:

在设计用于玻璃体内给药的药物递送剂时,可将亚10纳米大小的纳米载体的表面功能调整为靶向外部视网膜。
与年龄有关的黄斑变性(AMD)是不可逆性失明的主要原因之一,通常会影响工业化国家中50岁以上的人们。尽管抗血管内皮生长因子(VEGF)疗法在减缓新血管的生长方面有效,但这种复杂疾病很少能在视觉上有所改善。此外,目前每月玻璃体内重复注射药物的方案可能导致严重的副作用。迫切需要具有延长的治疗效果和有效递送至预期部位的联合疗法,以单独补充抗VEGF,这可以通过使用精心设计的纳米载体来实现。了解理化作用(例如玻璃体内施用的纳米载体的生物利用度,分布和在视网膜多层上的靶向效率)的大小,电荷,几何形状),这里我们制备了7种不同类型的表面官能化水溶性树突状纳米载体,其流体动力学尺寸大多在5 nm以下。将相似化学计算量的荧光团共价连接到这些生物相容性纳米载体中的每一个上,以便通过冷冻切片的健康小鼠眼睛的共聚焦显微镜进行定量分析。有趣的是,在注射后24小时,表面上具有多个葡糖胺(D NSG)的纳米载体主要聚集在感光层和视网膜色素上皮(RPE)中,这被认为与AMD的发病机制有关(即,目标网站)。此外,D NSG证明了在这些视网膜外层的延长停留时间,似乎可以通过聚集逐渐变成微米级的颗粒。我们的系统研究结果可能为玻璃体内纳米载体的合理设计提供有用的指导,以治疗威胁视力的视网膜疾病,包括AMD。


























 京公网安备 11010802027423号
京公网安备 11010802027423号