当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tunable LiCl@UiO-66 composites for water sorption-based heat transformation applications
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03442h Yangyang Sun 1, 2, 3, 4 , Alex Spieß 1, 2, 3, 4 , Christian Jansen 1, 2, 3, 4 , Alexander Nuhnen 1, 2, 3, 4 , Serkan Gökpinar 1, 2, 3, 4 , Raphael Wiedey 2, 3, 4, 5 , Sebastian-Johannes Ernst 4, 6, 7 , Christoph Janiak 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-12 , DOI: 10.1039/d0ta03442h Yangyang Sun 1, 2, 3, 4 , Alex Spieß 1, 2, 3, 4 , Christian Jansen 1, 2, 3, 4 , Alexander Nuhnen 1, 2, 3, 4 , Serkan Gökpinar 1, 2, 3, 4 , Raphael Wiedey 2, 3, 4, 5 , Sebastian-Johannes Ernst 4, 6, 7 , Christoph Janiak 1, 2, 3, 4
Affiliation
|
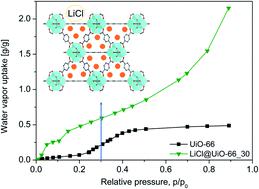
Porous composite materials are potential candidates for water-based adsorptive heat transformation (AHT) applications. Here, a solid adsorbent LiCl@UiO-66 as a 'composite salt inside porous matrix’ (CSPM) has been prepared by incorporating hygroscopic lithium chloride into a microporous metal–organic framework (MOF) UiO-66 as a host matrix through the wet impregnation method. In our wet impregnation we did not let the excess salt solution dry to prevent salt precipitation on the matrix surface. This yielded a true salt@MOF composite with no deliquescence of LiCl and strongly enhanced the water adsorption capacity of UiO-66 through the salt content. At p/p0 = 0.1 the water vapor sorption isotherms show a hydration state of LiCl inside the MOF of LiCl·2–4H2O which is much higher than for neat LiCl with 0.5H2O, due to the dispersion of a small particle size inside the matrix. LiCl@UiO-66 with a 30 wt% LiCl content (LiCl@UiO-66_30) has a 3 to 8 times higher water uptake over neat UiO-66 (depending on relative pressure) and could reach a volumetric and gravimetric water uptake of over 2.15 g g−1 at p/p0 = 0.9, which outperforms the so far known UiO-66-based composites. Cycling tests confirmed the hydrothermal stability of the LiCl@UiO-66 composites. Kinetic evaluation of the gravimetric water uptake (at 90% relative humidity) over time yielded rate coefficients up to 2.0(1) × 10−4 s−1 which is slower than that in neat UiO-66 (6.7(6) × 10−4 s−1) but faster than that for salt@silica gel composites. The coefficient of performance for the heat pumping mode (at Tdes/Tads/Tevap set to 90/40/10 °C) of 1.64 for LiCl@UiO-66_30 exceeds those of other MOFs, salt@MOF or salt@silica gel composites. For thermal battery applications the heat storage capacity (CHS) for LiCl@UiO-66_30 is 900 kJ kg−1 (=0.25 kW h kg−1), which can reach the Department of Energy (DOE) value of 2.5 kW h/35 kg with just 10 kg of material and outperforms CaCl2@UiO-66_38 with a CHS value of 367 kJ kg−1.
中文翻译:

可调节的LiCl @ UiO-66复合材料,用于基于水吸收的热转化应用
多孔复合材料是水基吸附热转化(AHT)应用的潜在候选材料。在这里,通过将吸湿性氯化锂通过湿法掺入作为主体基质的微孔金属-有机骨架(MOF)UiO-66中,制备了作为“多孔基质中的复合盐”的固体吸附剂LiCl @ UiO-66。浸渍方法。在湿法浸渍中,我们没有让过量的盐溶液干燥以防止盐在基质表面上沉淀。这样制得的是真正的Salt @ MOF复合材料,没有LiCl的潮解作用,并且通过含盐量大大增强了UiO-66的吸水能力。在p / p 0 = 0.1时,水蒸气吸附等温线显示LiCl·2-4H 2 MOF内LiCl的水合状态由于基体内有小粒径的分散,O比含0.5H 2 O的纯LiCl高得多。LiCl含量为30 wt%的LiCl @ UiO-66(LiCl @ UiO-66_30)的吸水量是纯UiO-66的3至8倍(取决于相对压力),体积和重量吸水量可超过纯UiO-66。在p / p 0 = 0.9时为2.15 gg -1,优于目前已知的基于UiO-66的复合材料。循环测试证实了LiCl @ UiO-66复合材料的水热稳定性。重量吸水量(相对湿度为90%)随时间的动力学评估得出速率系数高达2.0(1)×10 -4 s -1比纯UiO-66(6.7(6)×10 -4 s -1)慢,但比盐@硅胶复合材料快。的表现为热泵送模式(在系数Ť DES / Ť广告/ Ť EVAP至90/40/10集℃)的1.64的LiCl @ UIO-66_30超过那些其他的MOF,盐@ MOF或盐@二氧化硅凝胶复合材料。对于热电池应用,LiCl @ UiO-66_30的储热容量(C HS)为900 kJ kg -1(= 0.25 kW h kg -1),可以达到能源部(DOE)的2.5 kW h / 35公斤(仅10公斤物料),性能优于CaCl 2@ UiO-66_38,其C HS值为367 kJ kg -1。
更新日期:2020-07-07
中文翻译:

可调节的LiCl @ UiO-66复合材料,用于基于水吸收的热转化应用
多孔复合材料是水基吸附热转化(AHT)应用的潜在候选材料。在这里,通过将吸湿性氯化锂通过湿法掺入作为主体基质的微孔金属-有机骨架(MOF)UiO-66中,制备了作为“多孔基质中的复合盐”的固体吸附剂LiCl @ UiO-66。浸渍方法。在湿法浸渍中,我们没有让过量的盐溶液干燥以防止盐在基质表面上沉淀。这样制得的是真正的Salt @ MOF复合材料,没有LiCl的潮解作用,并且通过含盐量大大增强了UiO-66的吸水能力。在p / p 0 = 0.1时,水蒸气吸附等温线显示LiCl·2-4H 2 MOF内LiCl的水合状态由于基体内有小粒径的分散,O比含0.5H 2 O的纯LiCl高得多。LiCl含量为30 wt%的LiCl @ UiO-66(LiCl @ UiO-66_30)的吸水量是纯UiO-66的3至8倍(取决于相对压力),体积和重量吸水量可超过纯UiO-66。在p / p 0 = 0.9时为2.15 gg -1,优于目前已知的基于UiO-66的复合材料。循环测试证实了LiCl @ UiO-66复合材料的水热稳定性。重量吸水量(相对湿度为90%)随时间的动力学评估得出速率系数高达2.0(1)×10 -4 s -1比纯UiO-66(6.7(6)×10 -4 s -1)慢,但比盐@硅胶复合材料快。的表现为热泵送模式(在系数Ť DES / Ť广告/ Ť EVAP至90/40/10集℃)的1.64的LiCl @ UIO-66_30超过那些其他的MOF,盐@ MOF或盐@二氧化硅凝胶复合材料。对于热电池应用,LiCl @ UiO-66_30的储热容量(C HS)为900 kJ kg -1(= 0.25 kW h kg -1),可以达到能源部(DOE)的2.5 kW h / 35公斤(仅10公斤物料),性能优于CaCl 2@ UiO-66_38,其C HS值为367 kJ kg -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号