当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-06-12 , DOI: 10.1002/sctm.19-0434 Jessica M Sun 1 , Geraldine Dawson 2 , Lauren Franz 2 , Jill Howard 2 , Colleen McLaughlin 1 , Bethany Kistler 1 , Barbara Waters-Pick 3 , Norin Meadows 1 , Jesse Troy 1 , Joanne Kurtzberg 1
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-06-12 , DOI: 10.1002/sctm.19-0434 Jessica M Sun 1 , Geraldine Dawson 2 , Lauren Franz 2 , Jill Howard 2 , Colleen McLaughlin 1 , Bethany Kistler 1 , Barbara Waters-Pick 3 , Norin Meadows 1 , Jesse Troy 1 , Joanne Kurtzberg 1
Affiliation
|
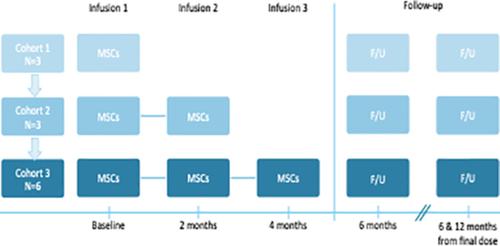
Ongoing neuroinflammation may contribute to symptoms of autism spectrum disorder (ASD) in at least a portion of affected individuals. Mesenchymal stromal cells (MSCs) have demonstrated the capacity to modulate neuroinflammation, but safety and feasibility of MSC administration in children with ASD have not been well established. In this open‐label, phase I study, 12 children with ASD between 4 and 9 years of age were treated with intravenous (IV) infusions of human cord tissue mesenchymal stromal cells (hCT‐MSCs), a third‐party MSC product manufactured from unrelated donor umbilical cord tissue. Children received one, two, or three doses of 2 × 106 cells per kilogram at 2‐month intervals. Clinical and laboratory evaluations were performed in person at baseline and 6 months and remotely at 12 months after the final infusion. Aside from agitation during the IV placement and infusion in some participants, hCT‐MSCs were well tolerated. Five participants developed new class I anti‐human leukocyte antigen (HLA) antibodies, associated with a specific lot of hCT‐MSCs or with a partial HLA match between donor and recipient. These antibodies were clinically silent and not associated with any clinical manifestations to date. Six of 12 participants demonstrated improvement in at least two ASD‐specific measures. Manufacturing and administration of hCT‐MSCs appear to be safe and feasible in young children with ASD. Efficacy will be evaluated in a subsequent phase II randomized, placebo‐controlled clinical trial.
中文翻译:

自闭症谱系障碍儿童人脐带组织间充质基质细胞的输注。
在至少一部分受影响的个体中,持续的神经炎症可能导致自闭症谱系障碍 (ASD) 的症状。间充质基质细胞 (MSC) 已证明具有调节神经炎症的能力,但尚未完全确定 MSC 在 ASD 儿童中给药的安全性和可行性。在这项开放标签的 I 期研究中,12 名 4 至 9 岁的 ASD 儿童接受了静脉 (IV) 输注人脐带组织间充质基质细胞 (hCT-MSCs) 的治疗,这是一种由无关的供体脐带组织。儿童接受一剂、两剂或三剂 2 × 10 6每公斤细胞每 2 个月一次。在基线和 6 个月时亲自进行临床和实验室评估,并在最后一次输注后 12 个月远程进行。除了一些参与者在 IV 放置和输注过程中的躁动外,hCT-MSC 的耐受性良好。五名参与者开发了新的 I 类抗人白细胞抗原 (HLA) 抗体,与特定批次的 hCT-MSC 或供体和受体之间的部分 HLA 匹配相关。这些抗体在临床上是沉默的,并且与迄今为止的任何临床表现无关。12 名参与者中有 6 名在至少两项 ASD 特定措施方面表现出改善。在患有 ASD 的幼儿中,hCT-MSCs 的制造和管理似乎是安全可行的。疗效将在随后的 II 期随机、
更新日期:2020-06-12
中文翻译:

自闭症谱系障碍儿童人脐带组织间充质基质细胞的输注。
在至少一部分受影响的个体中,持续的神经炎症可能导致自闭症谱系障碍 (ASD) 的症状。间充质基质细胞 (MSC) 已证明具有调节神经炎症的能力,但尚未完全确定 MSC 在 ASD 儿童中给药的安全性和可行性。在这项开放标签的 I 期研究中,12 名 4 至 9 岁的 ASD 儿童接受了静脉 (IV) 输注人脐带组织间充质基质细胞 (hCT-MSCs) 的治疗,这是一种由无关的供体脐带组织。儿童接受一剂、两剂或三剂 2 × 10 6每公斤细胞每 2 个月一次。在基线和 6 个月时亲自进行临床和实验室评估,并在最后一次输注后 12 个月远程进行。除了一些参与者在 IV 放置和输注过程中的躁动外,hCT-MSC 的耐受性良好。五名参与者开发了新的 I 类抗人白细胞抗原 (HLA) 抗体,与特定批次的 hCT-MSC 或供体和受体之间的部分 HLA 匹配相关。这些抗体在临床上是沉默的,并且与迄今为止的任何临床表现无关。12 名参与者中有 6 名在至少两项 ASD 特定措施方面表现出改善。在患有 ASD 的幼儿中,hCT-MSCs 的制造和管理似乎是安全可行的。疗效将在随后的 II 期随机、



























 京公网安备 11010802027423号
京公网安备 11010802027423号