Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.tetlet.2020.152129 Mrinal K. Das , Abhinay Yadav , Satyajit Majumder , Alakesh Bisai
|
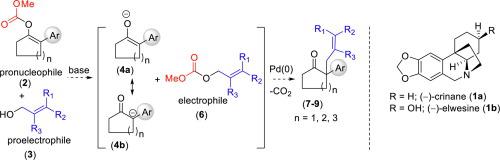
An efficient Pd(0)-catalyzed deacylative allylation (DaA) of enolcarbonates (as pro-nucleophile) of cycloalkanones sharing acyl functionality at C2-position with readily available allylic alcohols (as pro-electrophiles) is disclosed under mild reaction conditions. A wide variety of cycloalkanones with an aromatic ring and allyl group at C-2 position (all-carbon quaternary center) are obtained in good to excellent yields (36 examples). The usefulness of this methodology has been shown by a total synthesis of Amaryllidaceae alkaloid, (±)-crinane from 2-aryl cyclohexanone in 5 steps.
中文翻译:

烯醇碳酸盐的催化脱酰基烷基化(DaA):(±)-三氢呋喃的全合成
在温和的反应条件下,公开了一种有效的Pd(0)催化的环烷酮的烯醇碳酸酯(作为亲核体)的Pd(0)催化脱酰基烯丙基化(DaA),该环烷酮在C 2位与容易获得的烯丙醇(作为亲电子体)共享酰基功能。获得了各种具有良好至极好的收率的,具有在C-2位置(全碳季中心)的芳环和烯丙基的环烷酮(36个实例)。该方法的有用性已由5个步骤由2-芳基环己酮全合成金莲花科生物碱,(±)-肉桂烷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号